Mrgprb2-dependent Mast Cell Activation Plays a Crucial Role in Acute Colitis
- PMID: 39179175
- PMCID: PMC11462171
- DOI: 10.1016/j.jcmgh.2024.101391
Mrgprb2-dependent Mast Cell Activation Plays a Crucial Role in Acute Colitis
Abstract
Background & aims: Mast cells (MCs) are typically found at mucosal surfaces, where their immunoglobulin E (IgE)-dependent activation plays a central role in allergic diseases. Over the past years, signaling through Mas-related G protein-coupled receptor b2 (Mrgprb2) in mice and MRGPRX2 in humans has gained a lot of interest as an alternative MC activation pathway with high therapeutic potential. The aim of this study was to explore the relevance of such IgE-independent, Mrgprb2-mediated signaling in colonic MCs in the healthy and acutely inflamed mouse colon.
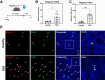
Methods: Mrgprb2 expression and functionality was studied using a genetic labeling strategy combined with advanced microscopic imaging. Furthermore, Mrgprb2 knockout (Mrgprb2-/-) mice were used to determine the role of this pathway in a preclinical dextran sodium sulphate (DSS) colitis model.
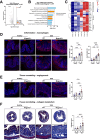
Results: We found that Mrgprb2 acts as a novel MC degranulation pathway in a large subset of connective tissue MCs in the mouse distal colon. Acute DSS colitis induced a pronounced increase of Mrgprb2-expressing MCs, which were found in close association with Substance P-positive nerve fibers. Loss of Mrgprb2-mediated signaling impaired DSS-induced neutrophil influx and significantly impacted on acute colitis progression.
Conclusions: Our findings uncover a novel, IgE-independent MC degranulation pathway in the mouse colon that plays a central role in acute colitis pathophysiology, mainly by safeguarding acute colitis progression and severity in mice. This pseudo allergic, Mrgprb2-induced signaling is part of a hitherto unconsidered colonic neuro-immune pathway and might have significant potential for the further development of effective therapeutic treatment strategies for gastrointestinal disorders, such as ulcerative colitis.
Keywords: Colitis; IgE-independent; Mas-related G Protein Coupled Receptor; Mast Cell.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Galli S.J., Gaudenzio N., Tsai M. Mast cells in inflammation and disease: recent progress and ongoing concerns. Annu Rev Immunol. 2020;38:49–77. - PubMed
-
- Elieh Ali Komi D., Wöhrl S., Bielory L. Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol. 2020;58:342–365. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

