Helminth protein enhances wound healing by inhibiting fibrosis and promoting tissue regeneration
- PMID: 39179288
- PMCID: PMC11342954
- DOI: 10.26508/lsa.202302249
Helminth protein enhances wound healing by inhibiting fibrosis and promoting tissue regeneration
Abstract
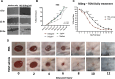
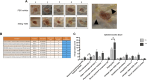
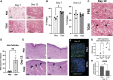
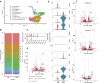
Skin wound healing due to full thickness wounds typically results in fibrosis and scarring, where parenchyma tissue is replaced with connective tissue. A major advance in wound healing research would be to instead promote tissue regeneration. Helminth parasites express excretory/secretory (ES) molecules, which can modulate mammalian host responses. One recently discovered ES protein, TGF-β mimic (TGM), binds the TGF-β receptor, though likely has other activities. Here, we demonstrate that topical administration of TGM under a Tegaderm bandage enhanced wound healing and tissue regeneration in an in vivo wound biopsy model. Increased restoration of normal tissue structure in the wound beds of TGM-treated mice was observed during mid- to late-stage wound healing. Both accelerated re-epithelialization and hair follicle regeneration were observed. Further analysis showed differential expansion of myeloid populations at different wound healing stages, suggesting recruitment and reprogramming of specific macrophage subsets. This study indicates a role for TGM as a potential therapeutic option for enhanced wound healing.
© 2024 Lothstein et al.
Conflict of interest statement
Rutgers, the State University of New Jersey has submitted a patent application on behalf of WC Gause, F Chen, Zhugong Liu, and P Mishra, which has been issue as Patent No. US 9,931,361 B2.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
