Evaluation of risk prediction models to select lung cancer screening participants in Europe: a prospective cohort consortium analysis
- PMID: 39179310
- PMCID: PMC11369914
- DOI: 10.1016/S2589-7500(24)00123-7
Evaluation of risk prediction models to select lung cancer screening participants in Europe: a prospective cohort consortium analysis
Abstract
Background: Lung cancer risk prediction models might efficiently identify individuals who should be offered lung cancer screening. However, their performance has not been comprehensively evaluated in Europe. We aimed to externally validate and evaluate the performance of several risk prediction models that predict lung cancer incidence or mortality in prospective European cohorts.
Methods: We analysed 240 137 participants aged 45-80 years with a current or former smoking history from nine European countries in four prospective cohorts from the pooled database of the Lung Cancer Cohort Consortium: the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (Finland), the Nord-Trøndelag Health Study (Norway), CONSTANCES (France), and the European Prospective Investigation into Cancer and Nutrition (Denmark, Germany, Italy, Spain, Sweden, the Netherlands, and Norway). We evaluated ten lung cancer risk models, which comprised the Bach, the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial 2012 model (PLCOm2012), the Lung Cancer Risk Assessment Tool (LCRAT), the Lung Cancer Death Risk Assessment Tool (LCDRAT), the Nord-Trøndelag Health Study (HUNT), the Optimized Early Warning Model for Lung Cancer Risk (OWL), the University College London-Death (UCLD), the University College London-Incidence (UCLI), the Liverpool Lung Project version 2 (LLP version 2), and the Liverpool Lung Project version 3 (LLP version 3) models. We quantified model calibration as the ratio of expected to observed cases or deaths and discrimination using the area under the receiver operating characteristic curve (AUC). For each model, we also identified risk thresholds that would screen the same number of individuals as each of the US Preventive Services Task Force 2021 (USPSTF-2021), the US Preventive Services Task Force 2013 (USPSTF-2013), and the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) criteria.
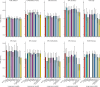
Findings: Among the participants, 1734 lung cancer cases and 1072 lung cancer deaths occurred within five years of enrolment. Most models had reasonable calibration in most countries, although the LLP version 2 overpredicted risk by more than 50% in eight countries (expected to observed ≥1·50). The PLCOm2012, LCDRAT, LCRAT, Bach, HUNT, OWL, UCLD, and UCLI models showed similar discrimination in most countries, with AUCs ranging from 0·68 (95% CI 0·59-0·77) to 0·83 (0·78-0·89), whereas the LLP version 2 and LLP version 3 showed lower discrimination, with AUCs ranging from 0·64 (95% CI 0·57-0·72) to 0·78 (0·74-0·83). When pooling data from all countries (but excluding the HUNT cohort), 33·9% (73 313 of 216 387) of individuals were eligible by USPSTF-2021 criteria, which included 74·8% (1185) of lung cancers and 76·3% (730) of lung cancer deaths occurring over 5 years. Fewer individuals were selected by USPSTF-2013 and NELSON criteria. After applying thresholds to select a population of equal size to USPSTF-2021, the PLCOm2012, LCDRAT, LCRAT, Bach, HUNT, OWL, UCLD, and UCLI, models identified 77·6%-79·1% of future cases, although they selected slightly older individuals compared with USPSTF-2021 criteria. Results were similar for USPSTF-2013 and NELSON.
Interpretation: Several lung cancer risk prediction models showed good performance in European countries and might improve the efficiency of lung cancer screening if used in place of categorical eligibility criteria.
Funding: US National Cancer Institute, l'Institut National du Cancer, Cancer Research UK.
This is an Open Access article published under the CC BY NC ND 3.0 IGO license which permits users to download and share the article for non-commercial purposes, so long as the article is reproduced in the whole without changes, and provided the original source is properly cited. This article shall not be used or reproduced in association with the promotion of commercial products, services or any entity. There should be no suggestion that WHO endorses any specific organisation, products or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Conflict of interest statement
Declaration of interests We declare no competing interests. Where authors are identified as personnel of the International Agency for Research on Cancer or WHO, the authors alone are responsible for the views expressed in this Article and they do not necessarily represent the decisions, policy, or views of those organisations.
Figures


References
-
- Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax. 2017;72:825–831. - PubMed
-
- Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening—results from the randomized German LUSI trial. Int J Cancer. 2020;146:1503–1513. - PubMed
-
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

