NLR stability predicts response to immune checkpoint inhibitors in advanced hepatocellular carcinoma
- PMID: 39179639
- PMCID: PMC11344071
- DOI: 10.1038/s41598-024-68048-9
NLR stability predicts response to immune checkpoint inhibitors in advanced hepatocellular carcinoma
Abstract
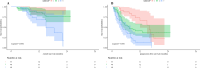
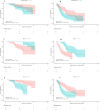
A high baseline NLR is associated with a poor prognosis of immunotherapy in patients with advanced HCC. As anti-tumour immune activation takes time, early dynamic changes in NLR may serve as a biomarker for predicting immunotherapy response. We conducted a retrospective study in which we enrolled 209 patients with aHCC who received ICIs (training cohort: N = 121, validation cohort: N = 88). In the training cohort, we categorized the patients based on the early changes in their NLR. Specifically, we defined patients as NLR Stable-Responder, NLR Responder and NLR Non-Responder. We compared the outcomes of these three patient groups using survival analysis. Additionally, we shortened the observation period to 6 weeks and validated the findings in the validation cohort. In the training cohort, early dynamic changes in NLR (HR 0.14, 95%CI 0.03-0.65, p = 0.012, HR 0.19, 95%CI 0.07-0.54, p = 0.002; HR 0.21, 95%CI 0.10-0.42, p < 0.001, HR 0.40, 95%CI 0.23-0.69, p = 0.001), PD-L1 < 1% (HR 5.36, 95%CI 1.12-25.66, p = 0.036; HR 2.98, 95%CI 1.51-5.91, p = 0.002) and MVI (HR 3.52, 95%CI 1.28-9.69, p = 0.015; HR 1.99, 95%CI 1.14-3.47, p = 0.015) were identified as independent predictors of OS and PFS. In the validation cohort, when the observation period was reduced to 6 weeks, early NLR changes still have predictive value. Early dynamic changes in NLR may be an easily defined, cost-effective, non-invasive biomarker to predict aHCC response to ICIs.
Keywords: Immunotherapy; NLR stability; OS; PD-L1; aHCC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Zhu, A. X., Finn, R. S., Edeline, J., Cattan, S., Ogasawara, S., Palmer, D., Verslype, C., Zagonel, V., Fartoux, L., Vogel, A., Sarker, D., Verset, G., Chan, S. L., Knox, J., Daniele, B., Webber, A. L., Ebbinghaus, S. W., Ma, J., Siegel, A. B., Cheng, A. L., Kudo, M. & KEYNOTE-224 Investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 19(7):940–952 (2018). Erratum in: Lancet Oncol. 19(9):e440 (2018). - PubMed
-
- Yau, T., Kang, Y. K., Kim, T. Y., El-Khoueiry, A. B., Santoro, A., Sangro, B., Melero, I., Kudo, M., Hou, M. M., Matilla, A., Tovoli, F., Knox, J. J., Ruth He, A., El-Rayes, B. F., Acosta-Rivera, M., Lim, H. Y., Neely, J., Shen, Y., Wisniewski, T., Anderson, J. & Hsu, C. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 6(11):e204564. 10.1001/jamaoncol.2020.4564 (2020). Erratum in: JAMA Oncol. 7(1):140 (2021). - PMC - PubMed
-
- Lee, M. S., Ryoo, B. Y., Hsu, C. H., Numata, K., Stein, S., Verret, W., Hack, S. P., Spahn, J., Liu, B., Abdullah, H., Wang, Y., He, A. R., Lee, K. H. & GO30140 Investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 21(6):808–820 (2020). Erratum in: Lancet Oncol. 21(7):e341 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

