Gelsolin alleviates rheumatoid arthritis by negatively regulating NLRP3 inflammasome activation
- PMID: 39179640
- PMCID: PMC11618363
- DOI: 10.1038/s41418-024-01367-6
Gelsolin alleviates rheumatoid arthritis by negatively regulating NLRP3 inflammasome activation
Abstract
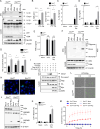
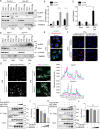
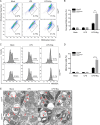
Despite numerous biomarkers being proposed for rheumatoid arthritis (RA), a gap remains in our understanding of their mechanisms of action. In this study, we discovered a novel role for gelsolin (GSN), an actin-binding protein whose levels are notably reduced in the plasma of RA patients. We elucidated that GSN is a key regulator of NLRP3 inflammasome activation in macrophages, providing a plausible explanation for the decreased secretion of GSN in RA patients. We found that GSN interacts with NLRP3 in LPS-primed macrophages, hence modulating the formation of the NLRP3 inflammasome complex. Reducing GSN expression significantly enhanced NLRP3 inflammasome activation. GSN impeded NLRP3 translocation to the mitochondria; it contributed to the maintenance of intracellular calcium equilibrium and mitochondrial stability. This maintenance is crucial for controlling the inflammatory response associated with RA. Furthermore, the exacerbation of arthritic symptoms in GSN-deficient mice indicates the potential of GSN as both a diagnostic biomarker and a therapeutic target. Moreover, not limited to RA models, GSN has demonstrated a protective function in diverse disease models associated with the NLRP3 inflammasome. Myeloid cell-specific GSN-knockout mice exhibited aggravated inflammatory responses in models of MSU-induced peritonitis, folic acid-induced acute tubular necrosis, and LPS-induced sepsis. These findings suggest novel therapeutic approaches that modulate GSN activity, offering promise for more effective management of RA and a broader spectrum of inflammatory conditions.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: This study did not involve any human participants. Mouse studies were conducted in accordance with the protocols approved by the Department of Laboratory Animal Resources Ethical Committee of Yonsei University College of Medicine and the President of Nippon Medical School.
Figures




References
MeSH terms
Substances
Grants and funding
- NRF-2023R1A2C1005804/National Research Foundation of Korea (NRF)
- RS-2024-00408822/National Research Foundation of Korea (NRF)
- NRF- 2022M3A9B6017424/National Research Foundation of Korea (NRF)
- 16H05178/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K07140/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

