High-dimensional cortical signals reveal rich bimodal and working memory-like representations among S1 neuron populations
- PMID: 39179675
- PMCID: PMC11344095
- DOI: 10.1038/s42003-024-06743-z
High-dimensional cortical signals reveal rich bimodal and working memory-like representations among S1 neuron populations
Abstract
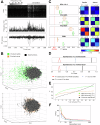
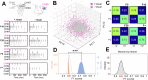
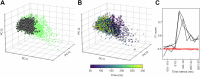
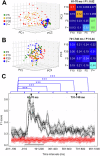
Complexity is important for flexibility of natural behavior and for the remarkably efficient learning of the brain. Here we assessed the signal complexity among neuron populations in somatosensory cortex (S1). To maximize our chances of capturing population-level signal complexity, we used highly repeatable resolvable visual, tactile, and visuo-tactile inputs and neuronal unit activity recorded at high temporal resolution. We found the state space of the spontaneous activity to be extremely high-dimensional in S1 populations. Their processing of tactile inputs was profoundly modulated by visual inputs and even fine nuances of visual input patterns were separated. Moreover, the dynamic activity states of the S1 neuron population signaled the preceding specific input long after the stimulation had terminated, i.e., resident information that could be a substrate for a working memory. Hence, the recorded high-dimensional representations carried rich multimodal and internal working memory-like signals supporting high complexity in cortical circuitry operation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures

Similar articles
-
Cross-modal involvement of the primary somatosensory cortex in visual working memory: A repetitive TMS study.Neurobiol Learn Mem. 2020 Nov;175:107325. doi: 10.1016/j.nlm.2020.107325. Epub 2020 Oct 12. Neurobiol Learn Mem. 2020. PMID: 33059033
-
Neuronal activity of somatosensory cortex in a cross-modal (visuo-haptic) memory task.Exp Brain Res. 1997 Oct;116(3):551-5. doi: 10.1007/pl00005783. Exp Brain Res. 1997. PMID: 9372304
-
Cortical and thalamic contributions to response dynamics across layers of the primary somatosensory cortex during tactile discrimination.J Neurophysiol. 2015 Sep;114(3):1652-76. doi: 10.1152/jn.00108.2015. Epub 2015 Jul 15. J Neurophysiol. 2015. PMID: 26180115 Free PMC article.
-
Distributed neural networks of tactile working memory.J Physiol Paris. 2013 Dec;107(6):452-8. doi: 10.1016/j.jphysparis.2013.06.001. Epub 2013 Jun 17. J Physiol Paris. 2013. PMID: 23792021 Free PMC article. Review.
-
Attending to and remembering tactile stimuli: a review of brain imaging data and single-neuron responses.J Clin Neurophysiol. 2000 Nov;17(6):575-91. doi: 10.1097/00004691-200011000-00004. J Clin Neurophysiol. 2000. PMID: 11151976 Review.
References
-
- Cavanaugh, J. T., Kelty-Stephen, D. G. & Stergiou, N. Multifractality, interactivity, and the adaptive capacity of the human movement system: a perspective for advancing the conceptual basis of neurologic physical therapy. J. Neurol. Phys. Ther.41, 245–251 (2017). 10.1097/NPT.0000000000000199 - DOI - PMC - PubMed
-
- Li, Z. et al. An indicator to quantify the complexity of signals and surfaces based on scaling behaviors transcending fractal. Chaos Solitons Fractals 163. 10.1016/j.chaos.2022.112556 (2022).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

