Targeting MERTK on tumour cells and macrophages: a potential intervention for sporadic and NF2-related meningioma and schwannoma tumours
- PMID: 39179860
- PMCID: PMC11458476
- DOI: 10.1038/s41388-024-03131-z
Targeting MERTK on tumour cells and macrophages: a potential intervention for sporadic and NF2-related meningioma and schwannoma tumours
Erratum in
-
Correction: Targeting MERTK on tumour cells and macrophages: a potential intervention for sporadic and NF2-related meningioma and schwannoma tumours.Oncogene. 2024 Oct;43(41):3080. doi: 10.1038/s41388-024-03177-z. Oncogene. 2024. PMID: 39327460 Free PMC article. No abstract available.
Abstract
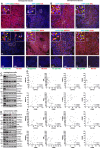
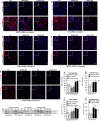
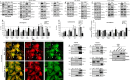
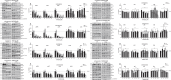
Meningioma and schwannoma are common tumours of the nervous system. They occur sporadically or as part of the hereditary NF2-related schwannomatosis syndrome. There is an unmet need for new effective drug treatments for both tumour types. In this paper, we demonstrate overexpression/activation of TAM (TYRO3/AXL/MERTK) receptors (TAMs) and overexpression/release of ligand GAS6 in patient-derived meningioma tumour cells and tissue. For the first time, we reveal the formation of MERTK/TYRO3 heterocomplexes in meningioma and schwannoma tissue. We demonstrate the dependence of AXL and TYRO3 expression on MERTK in both tumour types, as well as interdependency of MERTK and AXL expression in meningioma. We show that MERTK and AXL contribute to increased proliferation and survival of meningioma and schwannoma cells, which we inhibited in vitro using the MERTK/FLT3 inhibitor UNC2025 and the AXL inhibitor BGB324. UNC2025 was effective in both tumour types with superior efficacy over BGB324. Finally, we found that TAMs are expressed by tumour-associated macrophages in meningioma and schwannoma tumours and that UNC2025 strongly depleted macrophages in both tumour types.
© 2024. he Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Plotkin SR, Messiaen L, Legius E, Pancza P, Avery RA, Blakeley JO, et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet Med. 2022;24:1967–77. - PubMed
-
- Agnihotri S, Jalali S, Wilson MR, Danesh A, Li M, Klironomos G, et al. The genomic landscape of schwannoma. Nat Genet. 2016;48:1339–48. - PubMed
-
- Wang JZ, Nassiri F, Mawrin C, Zadeh G. Genomic Landscape of Meningiomas. Adv Exp Med Biol. 2023;1416:137–58. - PubMed
-
- Champeaux-Depond C, Weller J, Resche-Rigon M. Neurofibromatosis type 2: a nationwide population-based study focused on survival after meningioma surgery. Clin Neurol Neurosurg. 2020;198:106236. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

