Effects of Limosilactobacillus reuteri strains PTA-126787 and PTA-126788 on intestinal barrier integrity and immune homeostasis in an alcohol-induced leaky gut model
- PMID: 39179898
- PMCID: PMC11344072
- DOI: 10.1038/s41598-024-70549-6
Effects of Limosilactobacillus reuteri strains PTA-126787 and PTA-126788 on intestinal barrier integrity and immune homeostasis in an alcohol-induced leaky gut model
Abstract
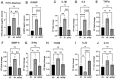
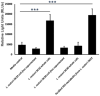
Intestinal barrier is a first line of defense that prevents entry of various harmful substances from the lumen into the systemic environment. Impaired barrier function with consequent translocation of harmful substances into systemic circulation ("leaky gut") is a central theme in many gastrointestinal, autoimmune, mental, and metabolic diseases. Probiotics have emerged as a promising strategy to maintain intestinal integrity and address "leaky gut". Using in silico, in vitro and avian in vivo analyses, we previously showed that two novel L. reuteri strains, PTA-126787 (L. reuteri 3630) and PTA-126788 (L. reuteri 3632), isolated from broiler chickens possess favorable safety profiles. Consistent with a recent study, here we show that L. reuteri 3630 and 3632 are phylogenetically similar to human L. reuteri strains. Daily administration of high doses of L. reuteri 3630 and 3632 to Sprague Dawley rats for 28 days was found to be safe with no adverse effects. More importantly, administration of L. reuteri 3630 and 3632 significantly reduced markers associated with alcohol-induced leaky gut, by downregulating inflammatory cytokines and upregulating anti-inflammatory cytokines in an alcohol model of leaky gut in mice. While L. reuteri 3630 cells and supernatant showed no activation, L. reuteri 3632 cells but not supernatant showed activation of AhR, a key transcription factor that regulates gut and immune homeostasis. L. reuteri 3630 is creamish white in morphology typical of Lactobacillus species and L. reuteri 3632 displays a unique orange pigmentation, which was stable even after passaging for 480 generations. We identified a rare polyketide biosynthetic gene cluster in L. reuteri 3632 that likely encodes for the orange-pigmented secondary metabolite. Similar to L. reuteri 3632 cells, the purified orange metabolite activated AhR. All together, these data provide evidence on the phylogenetic relatedness, safety, efficacy, and one of the likely mechanisms of action of L. reuteri 3630 and 3632 for potential probiotic applications to address "leaky gut" and associated pathologies in humans.
© 2024. The Author(s).
Conflict of interest statement
I have read the journal’s policy and want to declare the following conflicts of interest. The authors DG, GP, EBH, and AK are employees of BiomEdit, LLC. BiomEdit, LLC is a company that discovers and develops microbiome-based solutions for animal health. The authors OW and SPM are employees of Elanco Animal Health, Inc. Elanco Animal Health, Inc. is a company that develops, manufactures, and sells veterinary pharmaceuticals. The authors AG and JP are employees of or were employees of MicroMGx, LLC at the time this work was completed. MicroMGx, LLC is a company specialized in metabologenomics services. The authors AZ, MG and DAW are employees of or were employees of LSUHSC at the time this work was completed. LSUHSC is a public university focused on health sciences. These affiliations do not alter our adherence to Scientific Reports policies on sharing data and materials. The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

