A Novel Stem Cell Model to Study Preeclampsia Endothelial Dysfunction
- PMID: 39179924
- PMCID: PMC11438721
- DOI: 10.1007/s43032-024-01590-z
A Novel Stem Cell Model to Study Preeclampsia Endothelial Dysfunction
Abstract
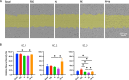
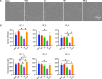
Preeclampsia is a common pregnancy complication affecting 5% to 7% of all pregnancies worldwide annually. While the pathogenesis is not fully understood, maternal endothelium dysfunction is thought to be a central component to preeclampsia development. Studies to dissect maternal endothelial dysfunction, particularly on a patient-specific basis, are hampered by limited access to systemic primary endothelial cells (ECs). The objective of this study was to establish a replenishable, patient-specific in vitro EC model to allow robust mechanistic studies to dissect endothelial dysfunction in preeclampsia. Induced pluripotent stem cells (iPSCs) from three women with a history of normotensive pregnancies were differentiated into ECs. The established ECs were exposed to pooled sera from normotensive pregnancies, preeclamptic pregnancies, normotensive postpartum for non-pregnant comparison and controls. Endothelial functions including nitric oxide (NO) release, cell migration, tube formation and viability were evaluated. Levels of NO release were significantly lower after incubation with preeclamptic sera compared to the fetal bovine serum (FBS) control, and normotensive and non-pregnant (postpartum) sera treatments were also lower than FBS but higher than preeclamptic sera treatments. Tube formation and cell migration were also impaired with preeclamptic sera compared to FBS controls. Cell viabilities remained unaffected by any sera treatment. Consistent outcomes were obtained across all three patient-specific lines treated with the same pooled sera. Establishment of patient-derived iPSC-ECs treated with pregnancy sera serves as a novel model to explore the interplay between individual maternal endothelial health and circulating factors that lead to endothelial dysfunction in preeclampsia.
Keywords: Endothelial dysfunction; Hypertension; Induced pluripotent stem cells (iPSCs); Preeclampsia; Pregnancy.
© 2024. The Author(s).
Conflict of interest statement
The authors have no financial interests that are directly or indirectly related to the work submitted for publication.
Figures



Similar articles
-
The role of soluble adhesion molecules in evaluating endothelial cell activation in preeclampsia.Am J Obstet Gynecol. 1999 Jan;180(1 Pt 1):68-72. doi: 10.1016/s0002-9378(99)70152-3. Am J Obstet Gynecol. 1999. PMID: 9914581
-
Altered Bioavailability of Nitric Oxide and L-Arginine Is a Key Determinant of Endothelial Dysfunction in Preeclampsia.Biomed Res Int. 2020 Oct 22;2020:3251956. doi: 10.1155/2020/3251956. eCollection 2020. Biomed Res Int. 2020. PMID: 33145345 Free PMC article.
-
Evaluation of nitric oxide as a mediator of severe preeclampsia.Am J Obstet Gynecol. 1996 Oct;175(4 Pt 1):1013-7. doi: 10.1016/s0002-9378(96)80044-5. Am J Obstet Gynecol. 1996. PMID: 8885767
-
[Endothelial dysfunction: role in the maternal syndrome of preeclampsia and long-term consequences for the cardiovascular system].Ann Cardiol Angeiol (Paris). 2013 Jun;62(3):215-20. doi: 10.1016/j.ancard.2013.03.002. Epub 2013 Apr 11. Ann Cardiol Angeiol (Paris). 2013. PMID: 23721989 Review. French.
-
[The role of endothelium in normal pregnancy and pregnancy complicated by preeclampsia].Ginekol Pol. 1999 Mar;70(3):113-9. Ginekol Pol. 1999. PMID: 10390912 Review. Polish.
References
-
- Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–112. 10.1161/CIRCRESAHA.118.313276. - PubMed
-
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–31. 10.1097/01.AOG.0000437382.03963.88. - PubMed
-
- Gestational Hypertension and Preeclampsia. ACOG practice bulletin, number 222. Obstet Gynecol. 2020;135:e237–60. 10.1097/AOG.0000000000003891. - PubMed
-
- Tanner MS, Davey M-A, Mol BW, Rolnik DL. The evolution of the diagnostic criteria of preeclampsia-eclampsia. Am J Obstet Gynecol. 2022;226:S835–43. 10.1016/j.ajog.2021.11.1371. - PubMed
MeSH terms
Substances
Grants and funding
- 75N92020D00019/HL/NHLBI NIH HHS/United States
- R01 HL158641/HL/NHLBI NIH HHS/United States
- R01 HL161002/HL/NHLBI NIH HHS/United States
- 10CRP3670014/American Heart Association South West Affiliates Clinical Research Grant
- K12 HDO57022/NIH Building Interdisciplinary Research Careers in Women's Health (BIRCWH)
LinkOut - more resources
Full Text Sources

