The progress of tumor vaccines clinical trials in non-small cell lung cancer
- PMID: 39179939
- PMCID: PMC11914286
- DOI: 10.1007/s12094-024-03678-z
The progress of tumor vaccines clinical trials in non-small cell lung cancer
Abstract
Background: Non-small cell lung cancer (NSCLC) remains a significant global health challenge, with high mortality rates and limited treatment options. Tumor vaccines have emerged as a potential therapeutic approach, aiming to stimulate the immune system to specifically target tumor cells.
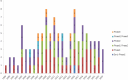
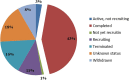
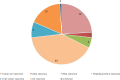
Methods: This study screened 283 clinical trials registered on ClinicalTrials.gov through July 31, 2023. After excluding data that did not meet the inclusion criteria, a total of 108 trials were assessed. Data on registered number, study title, study status, vaccine types, study results, conditions, interventions, outcome measures, sponsor, collaborators, drug target, phases, enrollment, start date, completion date and locations were extracted and analyzed.
Results: The number of vaccines clinical trials for NSCLC has continued to increase in recent years, the majority of which were conducted in the United States. Most of the clinical trials were at stages ranging from Phase I to Phase II. Peptide-based vaccines accounted for the largest proportion. Others include tumor cell vaccines, DNA/RNA vaccines, viral vector vaccines, and DC vaccines. Several promising tumor vaccine candidates have shown encouraging results in early-phase clinical trials. However, challenges such as heterogeneity of tumor antigens and immune escape mechanisms still need to be addressed.
Conclusion: Tumor vaccines represent a promising avenue in the treatment of NSCLC. Ongoing clinical trials are crucial for optimizing vaccine strategies and identifying the most effective combinations. Further research is needed to overcome existing limitations and translate these promising findings into clinical practice, offering new hope for NSCLC patients.
Keywords: Clinical trials; Immunotherapy; Non-small cell lung cancer (NSCLC); Studies; Tumor vaccines.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical