Higher phosphate concentrations as in aqueous humor of diabetic patients increase intraocular lens calcification
- PMID: 39179956
- PMCID: PMC11342483
- DOI: 10.1186/s12886-024-03553-z
Higher phosphate concentrations as in aqueous humor of diabetic patients increase intraocular lens calcification
Abstract
Background: Clinical evidence suggests an association between phosphate concentrations in aqueous humor and the risk of intraocular lens (IOL) calcification. To test this hypothesis the influence of different phosphate concentrations on IOL calcification was evaluated in an in vitro electrophoresis model.
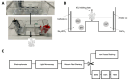
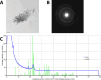
Methods: 20 IOLs of two hydrophilic IOL models (CT Spheris 204, Zeiss; Lentis L-313, Oculentis) and one hydrophobic control IOL model (Clareon CNA0T0, Alcon) were exposed to physiologic and elevated phosphate concentrations, similar to diabetic aqueous humor. IOL calcification was analyzed by alizarin red staining, von Kossa staining, scanning electron microscopy, energy dispersive x-ray spectroscopy and transmission electron microscopy with electron diffraction.
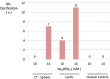
Results: Higher phosphate concentrations were associated with IOL calcification. Analyses of IOL surfaces and cross-sections documented calcification in no CT Spheris and 4 Lentis IOLs following exposure to 10 mM Na2HPO4, compared with 7 and 11 positive analyses following exposure to 14 mM Na2HPO4, respectively. Furthermore, a clear association between IOL calcification and the duration of electrophoresis was demonstrated, confirming increased phosphate concentrations and duration of exposure as risk factors of IOL calcification.
Conclusions: Findings suggest that higher phosphate concentrations in aqueous humor, as seen in diabetic patients, contribute to an increased IOL calcification risk, potentially explaining clinical observations showing an increased risk of IOL calcification in patients with diabetes.
Keywords: Aqueous humor; Diabetes; IOL Calcification; Phosphate concentration; Proliferative diabetic retinopathy.
© 2024. The Author(s).
Conflict of interest statement
R. Buhl, S. Schickhardt, L. Britz, and I. Lieberwirth declare that they have no competing interests. T. Yildirim reports personal fees from Alcon. R. Khoramnia reports grants, personal fees and non-financial support from Alimera, Alcon, Bayer, Johnson & Johnson, Hoya, Novartis, Physiol, Rayner and Roche, grants from Chengdu Kanghong, personal fees and non-financial support from Allergan, Kowa, Oculentis/Teleon, Oculus, Santen, and Acufocus. G. Auffarth reports grants, personal fees, non-financial support and consulting fees from Johnson & Johnson and Alcon, grants, personal fees and non-financial support from Carl Zeiss Meditec, Hoya, Kowa, Oculentis/Teleon, Rayner, Santen, Sifi, and Ursapharm, grants and personal fees from Biotech, Oculus, and EyeYon, grants from Acufocus, Anew, Contamac, Glaukos, Physiol, and Rheacell.
Figures






References
-
- Łabuz G, Yildirim TM, van den Berg T, et al. Assessment of straylight and the modulation transfer function of intraocular lenses with centrally localized opacification associated with the intraocular injection of gas. J Cataract Refract Surg. 2018;44:615–22. 10.1016/j.jcrs.2018.01.033. 10.1016/j.jcrs.2018.01.033 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

