Adverse events of tissue plasminogen activators in acute myocardial infarction patients: a real-world and pharmacovigilance database analysis
- PMID: 39179962
- PMCID: PMC11342586
- DOI: 10.1186/s12872-024-04121-5
Adverse events of tissue plasminogen activators in acute myocardial infarction patients: a real-world and pharmacovigilance database analysis
Abstract
Background: Tissue plasminogen activator (tPA) is recommended as the preferred thrombolytic therapy for acute myocardial infarction (AMI). This study aimed to explore tPA-related adverse events (AEs) reported in the United States Food and Drug Administration Adverse Event Reporting System (FAERS), assess the potential safety of three preferred tPA therapies for treating AMI, and provide guidance for selecting tPA for prehospital thrombolysis.
Method: Four algorithms, including ROR, PRR, BCPNN, and MGPS, were used to quantify the signals of Tenecteplase, Reteplase, and Alteplase related AEs and compare the differential degrees of the three tPA-associated AEs in the actual data.
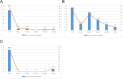
Result: We detected 18 signals of Tenecteplase-induced AE, 29 signals of Reteplase-induced AE, and 22 signals of Alteplase-induced AE. Among the three drugs, Tenecteplase had the highest signal intensity for intracranial hemorrhage-related AE, followed by Alteplase. Besides, Reteplase had the highest signal intensity for procedure-related AE and Alteplase had the highest signal intensity for arrhythmia-related AE. The time-onset analysis indicates that we should be vigilant for AEs, especially within the first week and the first 1-2 days after medication.
Conclusion: This study identified and compared the signals of AE related to Tenecteplase, Reteplase, and Alteplase in AMI patients.
Keywords: AMI; Alteplase; FAERS; Pharmacovigilance; Reteplase; Tenecteplase.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of Disease Study 2017 [published correction appears in Lancet. 2019;393(10190):e44. - PMC - PubMed
-
- Timmis A, Vardas P, Townsend N et al. European Society of Cardiology: cardiovascular disease statistics 2021 [published correction appears in. Eur Heart J. 2022.
-
- Members WC, Anderson HVS, Masri SC, et al. J Am Coll Cardiol. 2022;80(17):1660–700. 10.1016/j.jacc.2022.05.012. 2022 ACC/AHA Key Data Elements and Definitions for Chest Pain and Acute Myocardial Infarction: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Data Standards. 10.1016/j.jacc.2022.05.012 - DOI - PubMed
-
- Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. 10.1093/eurheartj/ehx393. 10.1093/eurheartj/ehx393 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

