Real-Life Use of Automated Insulin Delivery in Individuals With Type 2 Diabetes
- PMID: 39180292
- PMCID: PMC11572011
- DOI: 10.1177/19322968241274786
Real-Life Use of Automated Insulin Delivery in Individuals With Type 2 Diabetes
Abstract
Background: The objective of this work is to document performance of automated insulin delivery (AID) during real-life use in type 2 diabetes (T2D).
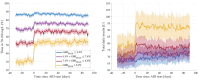
Methods: A retrospective analysis was performed of continuous glucose monitoring and insulin delivery data from 796 individuals with T2D, who transitioned from 1-month predictive low-glucose suspend (PLGS) use to 3-month AID use, in real-life settings. Primary outcome was change of time in range (TIR = 70-180 mg/dL) from PLGS to AID. Secondary outcomes included time above/below range (TAR/TBR) and total daily insulin (TDI).
Results: Compared with PLGS, AID increased TIR on average from 63.2% to 72.6%, decreased TAR from 36.2% to 26.8%, and increased TDI from 70.2 to 76.3 U (all P < .001), without significant change to TBR. Glycemic improvements were more pronounced in those with worse glycemic control during PLGS use (P < .001).
Conclusions: Real-life use of AID led to a rapid and sustained improvement of glycemic control in individuals with T2D.
Keywords: automated insulin delivery; continuous glucose monitoring; insulin pump; real life; time in range; type 2 diabetes.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.F. reports receiving patent royalties from Dexcom, Inc handled by the University of Virginia’s Licensing and Ventures Group. B.K. reports receiving research support from Dexcom, Inc and Tandem Diabetes Care handled by the University of Virginia; and patent royalties from Dexcom, Inc handled by the University of Virginia’s Licensing and Ventures Group. Tandem Diabetes Care provided the data analyzed in this work; Tandem Diabetes Care was not involved in the data analysis or interpretation of the results.
Figures
References
-
- Bergenstal RM, Garg S, Weinzimer SA, et al.. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407-1408. - PubMed
LinkOut - more resources
Full Text Sources

