CLOVER (CLOstridium difficile Vaccine Efficacy tRial) Study: A Phase 3, Randomized Trial Investigating the Efficacy and Safety of a Detoxified Toxin A/B Vaccine in Adults 50 Years and Older at Increased Risk of Clostridioides difficile Infection
- PMID: 39180325
- PMCID: PMC11650871
- DOI: 10.1093/cid/ciae410
CLOVER (CLOstridium difficile Vaccine Efficacy tRial) Study: A Phase 3, Randomized Trial Investigating the Efficacy and Safety of a Detoxified Toxin A/B Vaccine in Adults 50 Years and Older at Increased Risk of Clostridioides difficile Infection
Abstract
Background: Clostridioides difficile infection (CDI) causes substantial mortality and healthcare burden. We assessed the detoxified toxin-A/B PF-06425090 vaccine for primary CDI prevention.
Methods: This phase 3 observer-blinded study randomized (1:1) ≥50-year-olds at increased CDI risk (N = 17 535) to receive 3 PF-06425090 or placebo doses (0, 1, and 6 months). Primary end points were first CDI episode (≥3 unformed stools within 24 hours; central laboratory-confirmed toxin A/B positive) ≥14 days post-dose 3 (PD3; first primary) and post-dose 2 (PD2; second primary). CDI duration, need for CDI-related medical attention (secondary end points), and antibiotic use (post hoc analysis) PD3 were evaluated. Tolerability and safety were assessed.
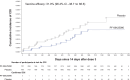
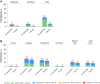
Results: The primary end point was not met (17 PF-06425090 and 25 placebo recipients had first CDI episode ≥14 days PD3 [vaccine efficacy (VE) = 31.0% (96.4% confidence interval [CI], -38.7% to 66.6%)]; 24 PF-06425090 and 34 placebo recipients had first CDI episode ≥14 days PD2 [VE = 28.6% (96.4% CI, -28.4% to 61.0%)]. Median CDI duration was lower with PF-06425090 (1 day) versus placebo (4 days; 2-sided nominal P = .02). Of participants with first CDI episode, 0 PF-06425090 and 11 placebo recipients sought CDI-related medical attention (post hoc analysis estimated VE = 100%; 95% CI, 59.6% to 100.0%) and 0 PF-06425090 and 10 placebo recipients required antibiotic treatment (VE = 100%; 95% CI, 54.8% to 100.0%). Local reactions were more frequent in PF-06425090 recipients, and systemic events were generally similar between groups; most were mild to moderate. Adverse event rates were similar between groups.
Conclusions: Three PF-06425090 doses were safe and well tolerated. Although the primary end point was not met, PF-06425090 reduced symptom duration, CDI that required medical attention, and CDI-directed antibiotic treatment, highlighting its potential to reduce CDI-associated healthcare burden.
Clinical trials registration: NCT03090191.
Keywords: Clostridioides difficile; Clostridioides difficile infection; adults; infectious disease; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. J. D. receives research support from Pfizer, Clorox, and Ecolab. E. R. D. receives research support from Theriva Biologics, Ferring, and Pfizer and is a consultant for Merck, Pfizer, Seres, Ferring, AstraZeneca, Abbott, Summit, and GSK. N. P. K. receives research support from Pfizer, Merck, Sanofi Pasteur, and GSK. E. G. L. receives research support from Pfizer. K. S. reports no conflicts of interest. M. H. W. has received consulting fees from Ferring, GSK, Nestlé, Paion, Pfizer, Phico Therapeutics, Qpex Biopharma, Seres, Summit, Tillotts, and Vedanta; lecture fees from GSK, Pfizer, Seres, and Tillotts; and grant support from GSK, Pfizer, Seres, Summit, the European Tissue Symposium, and Tillotts. J. L., S. B., H. Z., K. K., R. B., H. M. S., S. L., E. L., W. V. K., M. W. P. C. W., A. S. A., K. U. J., W. C. G., and N. K. are current or former employees of Pfizer and may hold stock or stock options. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Comment in
-
The End of Toxoid Vaccine Development for Preventing Clostridioides difficile Infections?Clin Infect Dis. 2024 Dec 17;79(6):1512-1514. doi: 10.1093/cid/ciae412. Clin Infect Dis. 2024. PMID: 39178347 No abstract available.
References
-
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372:1539–48. - PubMed
-
- Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994; 330:257–62. - PubMed
-
- Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clin Geriatr Med 2014; 30:79–93. - PubMed
-
- Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE; Shea Long-Term-Care Committee . Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol 2002; 23:696–703. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

