A novel index combining fecal immunochemical test, DNA test, and age improves detection of advanced colorectal adenoma
- PMID: 39180368
- PMCID: PMC11531960
- DOI: 10.1111/cas.16322
A novel index combining fecal immunochemical test, DNA test, and age improves detection of advanced colorectal adenoma
Abstract
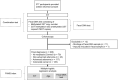
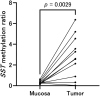
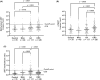
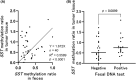
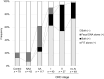
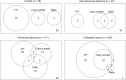
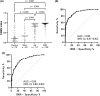
Although the fecal immunochemical test for hemoglobin (FIT) is a widely used screening test for colorectal cancer, it is not sensitive enough to detect advanced colorectal adenoma. To address this issue, we performed this study to investigate whether combining the FIT and fecal DNA testing of methylated somatostatin (SST) could improve diagnostic performance for advanced colorectal adenoma. We collected feces from 79 healthy subjects with negative results on colonoscopy, 43 patients with non-advanced colorectal adenoma, 117 patients with advanced colorectal adenoma, and 126 patients with colorectal cancer. After fecal DNA was incubated with methylation-sensitive restriction enzymes, SST methylation levels were measured by droplet digital PCR. Using logistic multivariate analysis, we established a prediction formula for detecting colorectal neoplasia and named it the FAMS (FIT, age, methylated SST) index. The diagnostic performance of a single use of FIT for advanced colorectal adenoma showed a sensitivity of 29.1% (34/117) and specificity of 89.3% (109/122). In contrast, the FAMS index showed a sensitivity of 56.4% (66/117) at a similar specificity point of 91.0% (111/122). Furthermore, even at the higher specificity point of 94.3% (115/122), the sensitivity was still higher than that of FIT, reaching 42.7% (50/117). As the FAMS index showed better diagnostic performance for advanced colorectal adenoma than a single use of FIT, the FAMS index could be a promising tool for detecting advanced colorectal adenoma.
Keywords: SST; advanced colorectal adenoma; fecal DNA test; fecal immunochemical test for hemoglobin; methylation.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Yutaka Suehiro and Takahiro Yamasaki received grants from Eiken Chemical. The other authors have no conflict of interest.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- Pawa N, Arulampalam T, Norton JD. Screening for colorectal cancer: established and emerging modalities. Nat Rev Gastroenterol Hepatol. 2011;8:711‐722. - PubMed
-
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal‐cancer screening. N Engl J Med. 2014;370:1287‐1297. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

