Structural insights into RNA cleavage by a novel family of bacterial RNases
- PMID: 39180400
- PMCID: PMC11417398
- DOI: 10.1093/nar/gkae717
Structural insights into RNA cleavage by a novel family of bacterial RNases
Abstract
Processing of RNA is a key regulatory mechanism for all living systems. Escherichia coli protein YicC belongs to the well-conserved YicC family and has been identified as a novel ribonuclease. Here, we report a 2.8-Å-resolution crystal structure of the E. coli YicC apo protein and a 3.2-Å-cryo-EM structure of YicC bound to an RNA substrate. The apo YicC forms a dimer of trimers with a large open channel. In the RNA-bound form, the top trimer of YicC rotates nearly 70° and closes the RNA substrate inside the cavity to form a clamshell-pearl conformation that resembles no other known RNases. The structural information combined with mass spectrometry and biochemical data identified cleavage on the upstream side of an RNA hairpin. Mutagenesis studies demonstrated that the previously uncharacterized domain, DUF1732, is critical in both RNA binding and catalysis. These studies shed light on the mechanism of the previously unexplored YicC RNase family.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
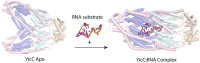




Update of
-
Structural insights into RNA cleavage by a novel family of bacterial RNases.Res Sq [Preprint]. 2023 Dec 27:rs.3.rs-3788707. doi: 10.21203/rs.3.rs-3788707/v1. Res Sq. 2023. Update in: Nucleic Acids Res. 2024 Sep 23;52(17):10705-10716. doi: 10.1093/nar/gkae717. PMID: 38234822 Free PMC article. Updated. Preprint.
References
-
- Poulsen P., Jensen K.F.. Three genes preceding pyrE on the Escherichia coli chromosome are essential for survival and normal cell morphology in stationary culture and at high temperature. Res. Microbiol. 1991; 142:283–288. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

