Squeezing Out Nanoparticles from Perovskites: Controlling Exsolution with Pressure
- PMID: 39180444
- PMCID: PMC11579965
- DOI: 10.1002/smll.202403544
Squeezing Out Nanoparticles from Perovskites: Controlling Exsolution with Pressure
Abstract
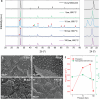
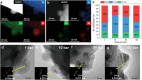
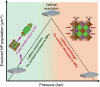
Nanoparticle exsolution has emerged as a versatile method to functionalize oxides with robust metallic nanoparticles for catalytic and energy applications. By modifying certain external parameters during thermal reduction (temperature, time, reducing gas), some morphological and/or compositional properties of the exsolved nanoparticles can be tuned. Here, it is shown how the application of high pressure (<100 bar H2) enables the control of the exsolution of ternary FeCoNi alloyed nanoparticles from a double perovskite. H2 pressure affects the lattice expansion and the nanoparticle characteristics (size, population, and composition). The composition of the alloyed nanoparticles could be controlled, showing a reversal of the expected thermodynamic trend at 10 and 50 bar, where Fe becomes the main component instead of Ni. In addition, pressure drastically lowers the exsolution temperature to 300 °C, resulting in unprecedented highly-dispersed and small-sized nanoparticles with a similar composition to those obtained at 600 °C and 10 bar. The mechanisms behind the effects of pressure on exsolution are discussed, involving kinetic, surface thermodynamics, and lattice-strain factors. A volcano-like trend of the exsolution extent suggests that competing pressure-dependent mechanisms govern the process. Pressure emerges as a new design tool for metallic nanoparticle exsolution enabling novel nanocatalysts and surface-functionalized materials.
Keywords: double perovskites; exsolution; metallic nanoparticles; pressure; ternary alloys.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Neagu D., Irvine J. T. S., Wang J., Yildiz B., Opitz A. K., Fleig J., Wang Y., Liu J., Shen L., Ciucci F., Rosen B. A., Xiao Y., Xie K., Yang G., Shao Z., Zhang Y., Reinke J., Schmauss T. A., Barnett S. A., Maring R., Kyriakou V., Mushtaq U., Tsampas M. N., Kim Y., O'Hayre R., Carrillo A. J., Ruh T., Lindenthal L., Schrenk F., Rameshan C., et al., J. Phys. Energy 2023, 5, 031501.
-
- Kousi K., Tang C., Metcalfe I. S., Neagu D., Small 2021, 17, 2006479. - PubMed
-
- Chen H., Sun Y., Yang S., Wang H., Dmowski W., Egami T., Dai S., Chem. Commun. 2020, 56, 15056. - PubMed
-
- Santaya M., Jiménez C. E., Troiani H. E., Carbonio E. A., Arce M. D., Toscani L. M., Garcia‐Diez R., Wilks R. G., Knop‐Gericke A., Bär M., Mogni L. V., J. Mater. Chem. A 2022, 10, 15554.
Grants and funding
LinkOut - more resources
Full Text Sources

