Applications of cell free protein synthesis in protein design
- PMID: 39180484
- PMCID: PMC11344276
- DOI: 10.1002/pro.5148
Applications of cell free protein synthesis in protein design
Abstract
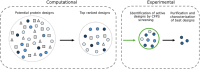
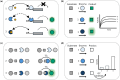
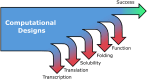
In protein design, the ultimate test of success is that the designs function as desired. Here, we discuss the utility of cell free protein synthesis (CFPS) as a rapid, convenient and versatile method to screen for activity. We champion the use of CFPS in screening potential designs. Compared to in vivo protein screening, a wider range of different activities can be evaluated using CFPS, and the scale on which it can easily be used-screening tens to hundreds of designed proteins-is ideally suited to current needs. Protein design using physics-based strategies tended to have a relatively low success rate, compared with current machine-learning based methods. Screening steps (such as yeast display) were often used to identify proteins that displayed the desired activity from many designs that were highly ranked computationally. We also describe how CFPS is well-suited to identify the reasons designs fail, which may include problems with transcription, translation, and solubility, in addition to not achieving the desired structure and function.
Keywords: cell‐free protein synthesis; medium throughput; protein design; screening; surface immobilization.
© 2024 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare that this review article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

