A novel KCND3 variant in the N-terminus impairs the ionic current of Kv4.3 and is associated with SCA19/22
- PMID: 39180521
- PMCID: PMC11344468
- DOI: 10.1111/jcmm.70039
A novel KCND3 variant in the N-terminus impairs the ionic current of Kv4.3 and is associated with SCA19/22
Abstract
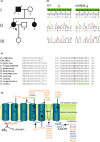
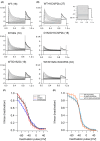
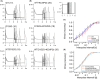
Spinocerebellar ataxias (SCAs) are a genetically heterogeneous group of autosomal dominant movement disorders. Among the SCAs associated with impaired ion channel function, SCA19/22 is caused by pathogenic variants in KCND3, which encodes the voltage-gated potassium channel Kv4.3. SCA19/22 is clinically characterized by ataxia, dysarthria and oculomotor dysfunction in combination with other signs and symptoms, including mild cognitive impairment, peripheral neuropathy and pyramidal signs. The known KCND3 pathogenic variants are localized either in the transmembrane segments, the connecting loops, or the C-terminal region of Kv4.3. We have identified a novel pathogenic variant, c.455A>G (p.D152G), localized in the N-terminus of Kv4.3. It is located in the immediate neighbourhood of the T1 domain, which is responsible for multimerization with the β-subunit KChIP2b and thus for the formation of functional heterooctamers. Electrophysiological studies showed that p.D152G does not affect channel gating, but reduces the ionic current in Kv4.3, even though the variant is not located in the transmembrane domains. Impaired channel trafficking to the plasma membrane may contribute to this effect. In a patient with a clinical picture corresponding to SCA19/22, p.D152G is the first pathogenic variant in the N-terminus of Kv4.3 to be described to date with an effect on ion channel activity.
Keywords: KCND3; Kv4.3; SCA19/22; ataxia; electrophysiology; movement disorder; neuropathology.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest.
Figures

References
-
- Klockgether T, Mariotti C, Paulson HL. Spinocerebellar ataxia. Nat Rev Dis Prim. 2019;5(1):24. - PubMed
-
- Coarelli G, Coutelier M, Durr A. Autosomal dominant cerebellar ataxias: new genes and progress towards treatments. Lancet Neurol. 2023;22(8):735‐749. - PubMed
-
- Perlman S. Hereditary ataxia overview. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews®. University of Washington, Seattle; 1998. - PubMed
-
- Waters MF, Minassian NA, Stevanin G, et al. Mutations in voltage‐gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet. 2006;38(4):447‐451. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

