The activity of therapeutic molecular cluster Ag5 is dependent on oxygen level and HIF-1 mediated signalling
- PMID: 39180984
- PMCID: PMC11388176
- DOI: 10.1016/j.redox.2024.103326
The activity of therapeutic molecular cluster Ag5 is dependent on oxygen level and HIF-1 mediated signalling
Abstract
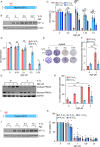
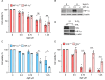
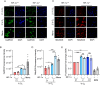
Regions of hypoxia occur in most solid tumours and are known to significantly impact therapy response and patient prognosis. Ag5 is a recently reported silver molecular cluster which inhibits both glutathione and thioredoxin signalling therefore limiting cellular antioxidant capacity. Ag5 treatment significantly reduces cell viability in a range of cancer cell lines with little to no impact on non-transformed cells. Characterisation of redox homeostasis in hypoxia demonstrated an increase in reactive oxygen species and glutathione albeit with different kinetics. Significant Ag5-mediated loss of viability was observed in a range of hypoxic conditions which mimic the tumour microenvironment however, this effect was reduced compared to normoxic conditions. Reduced sensitivity to Ag5 in hypoxia was attributed to HIF-1 mediated signalling to reduce PDH via PDK1/3 activity and changes in mitochondrial oxygen availability. Importantly, the addition of Ag5 significantly increased radiation-induced cell death in hypoxic conditions associated with radioresistance. Together, these data demonstrate Ag5 is a potent and cancer specific agent which could be used effectively in combination with radiotherapy.
Keywords: Ag5; HIF-1; Hypoxia; Radiation; Redox.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest RB is the CEO and Board Director of Arjuna Therapeutics. MT is the CSO of Arjuna Therapeutics. FD is scientific advisor and shareholder of Arjuna Therapeutics and has patents on Ag5 synthesis and the therapeutic applications.
Figures




References
-
- Vafa O., et al. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 2002;9:1031–1044. - PubMed
-
- Denko N. C. Hypoxia. HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer. 2008;8:705–713. - PubMed
-
- Hammond E.M., et al. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin. Oncol. 2014;26:277–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

