The significance of electrical signals in maturing spermatozoa for phosphoinositide regulation through voltage-sensing phosphatase
- PMID: 39181879
- PMCID: PMC11344830
- DOI: 10.1038/s41467-024-51755-2
The significance of electrical signals in maturing spermatozoa for phosphoinositide regulation through voltage-sensing phosphatase
Abstract
Voltage-sensing phosphatase (VSP) exhibits voltage-dependent phosphatase activity toward phosphoinositides. VSP generates a specialized phosphoinositide environment in mammalian sperm flagellum. However, the voltage-sensing mechanism of VSP in spermatozoa is not yet characterized. Here, we found that VSP is activated during sperm maturation, indicating that electric signals in immature spermatozoa are essential. Using a heterologous expression system, we show the voltage-sensing property of mouse VSP (mVSP). The voltage-sensing threshold of mVSP is approximately -30 mV, which is sensitive enough to activate mVSP in immature spermatozoa. We also report several knock-in mice in which we manipulate the voltage-sensitivity or electrochemical coupling of mVSP. Notably, the V312R mutant, with a minor voltage-sensitivity change, exhibits abnormal sperm motility after, but not before, capacitation. Additionally, the V312R mutant shows a significant change in the acyl-chain profile of phosphoinositide. Our findings suggest that electrical signals during sperm maturation are crucial for establishing the optimal phosphoinositide environment in spermatozoa.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



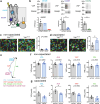

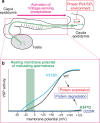
References
-
- Hille B. Ion channels of excitable membranes. Sinauer Associates Inc, Sunderland (2001).
MeSH terms
Substances
Grants and funding
- 20KK0376/MEXT | Japan Society for the Promotion of Science (JSPS)
- 17K15558/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20K07274/MEXT | Japan Society for the Promotion of Science (JSPS)
- 15H05901/MEXT | Japan Society for the Promotion of Science (JSPS)
- 21229003/MEXT | Japan Society for the Promotion of Science (JSPS)
- 25253016/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19H03401/MEXT | Japan Society for the Promotion of Science (JSPS)
- n.a/Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care (Ichiro Kanehara Foundation)
- n.a/Sumitomo Foundation
- n.a/Ono Medical Research Foundation
- n.a/Uehara Memorial Foundation
- n.a/Senri Life Science Foundation
- n.a/Takeda Science Foundation
- n.a/Mochida Memorial Foundation for Medical and Pharmaceutical Research
- 24gm1710007/Japan Agency for Medical Research and Development (AMED)
- JPMXP1323015483/Tokyo Medical and Dental University (TMDU)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

