WONDER-02: plastic stent vs. lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic pseudocysts-study protocol for a multicentre randomised non-inferiority trial
- PMID: 39182137
- PMCID: PMC11344301
- DOI: 10.1186/s13063-024-08373-6
WONDER-02: plastic stent vs. lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic pseudocysts-study protocol for a multicentre randomised non-inferiority trial
Abstract
Background: Endoscopic ultrasound (EUS)-guided transluminal drainage has become a first-line treatment modality for symptomatic pancreatic pseudocysts. Despite the increasing popularity of lumen-apposing metal stents (LAMSs), plastic stents may resolve non-necrotic fluid collections effectively with lower costs and no LAMS-specific adverse events. To date, there has been a paucity of data on the appropriate stent type in this setting. This trial aims to assess the non-inferiority of plastic stents to a LAMS for the initial EUS-guided drainage of pseudocysts.
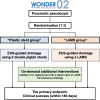
Methods: The WONDER-02 trial is a multicentre, open-label, non-inferiority, randomised controlled trial, which will enrol pancreatic pseudocyst patients requiring EUS-guided treatment in 26 centres in Japan. This trial plans to enrol 80 patients who will be randomised at a 1:1 ratio to receive either plastic stents or a LAMS (40 patients per arm). In the plastic stent group, EUS-guided drainage will be performed using two 7-Fr double pigtail stents. In the LAMS group, the treatment will be performed in the same way except for LAMS use. The step-up treatment will be performed via endoscopic and/or percutaneous procedures at the trial investigator's discretion. The primary endpoint is clinical success, which is defined as a decrease in a pseudocyst size to ≤ 2 cm and an improvement in inflammatory indicators (i.e. body temperature, white blood cell count, and serum C-reactive protein). Secondary endpoints include technical success, adverse events including mortality, pseudocyst recurrence, and medical costs.
Discussion: The WONDER-02 trial will investigate the efficacy and safety of plastic stents compared to a LAMS in EUS-guided treatment of symptomatic pancreatic pseudocysts with a particular focus on the non-inferior efficacy of plastic stents. The findings will help establish a new treatment algorithm for this population.
Trial registration: ClinicalTrials.gov NCT06133023 registered on 9 November 2023. UMIN000052647 registered on 30 October 2023. jRCT1032230444 registered on 7 November 2023.
Keywords: Drainage; Endoscopy; Endosonography; Mortality; Pancreatic fistula; Pancreatic pseudocyst; Pancreatitis; Randomised clinical trial; Sepsis; Stents.
© 2024. The Author(s).
Conflict of interest statement
H.I. declares research funding from Boston Scientific Japan, Fujifilm, and Piolax Medical Devices; honoraria from Boston Scientific Japan, Century Medical, Create Medic, Fujifilm, Gadelius Medical, Hitachi Medical, Japan Lifeline, Kaneka, Kawasumi Laboratories, Olympus Medical, Piolax Medical Devices, Sumitomo Bakelite, UMIDAS, and Zeon Medical; and contributions from Boston Scientific Japan, Gadelius Medical, Japan Lifeline, and Zeon Medical. Y.N. declares research funding from Boston Scientific Japan, Century Medical, Fujifilm, Gadelius Medical, HOYA Pentax Medical, Hitachi Medical, Kaneka, and Medico’s Hirata; and honoraria from Boston Scientific Japan, Fujifilm, Gadelius Medical, Hitachi Medical, J-MIT, Medico’s Hirata, and Olympus Medical. This work was not supported by any of those companies. The other authors declare no conflicts of interest related to this article.
Figures
References
-
- Iwashita T, Iwata K, Hamada T, Saito T, Shiomi H, Takenaka M, et al. Supportive treatment during the periprocedural period of endoscopic treatment for pancreatic fluid collections: a critical review of current knowledge and future perspectives. J Gastroenterol. 2023;58(2):98–111. 10.1007/s00535-022-01935-y - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials