Lentiviral vector gene therapy and CFTR modulators show comparable effectiveness in cystic fibrosis rat airway models
- PMID: 39183346
- PMCID: PMC11576507
- DOI: 10.1038/s41434-024-00480-y
Lentiviral vector gene therapy and CFTR modulators show comparable effectiveness in cystic fibrosis rat airway models
Abstract
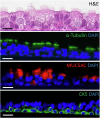
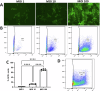
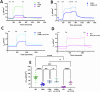
Mutation-agnostic treatments such as airway gene therapy have the potential to treat any individual with cystic fibrosis (CF), irrespective of their CF transmembrane conductance regulator (CFTR) gene variants. The aim of this study was to employ two CF rat models, Phe508del and CFTR knockout (KO), to assess the comparative effectiveness of CFTR modulators and lentiviral (LV) vector-mediated gene therapy. Cells were isolated from the tracheas of rats and used to establish air-liquid interface (ALI) cultures. Phe508del rat ALIs were treated with the modulator combination, elexacaftor-tezacaftor-ivacaftor (ETI), and separate groups of Phe508del and KO tracheal epithelial cells were treated with LV-CFTR followed by differentiation at ALI. Ussing chamber measurements were performed to assess CFTR function. ETI-treated Phe508del ALI cultures demonstrated CFTR function that was 59% of wild-type level, while gene-addition therapy restored Phe508del to 68% and KO to 47% of wild-type level, respectively. Our findings show that rat Phe508del-CFTR protein can be successfully rescued with ETI treatment, and that CFTR gene-addition therapy provides significant CFTR correction in Phe508del and KO ALI cultures to levels that were comparable to ETI. These findings highlight the potential of an LV vector-based gene therapy for the treatment of CF lung disease.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Turcios NL. Cystic fibrosis lung disease: an overview. Respir Care. 2020;65:233–51. - PubMed
-
- Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36:1–7. - PubMed
-
- McCarron A, Cmielewski P, Reyne N, McIntyre C, Finnie J, Craig F, et al. Phenotypic characterization and comparison of cystic fibrosis rat models generated using CRISPR/Cas9 gene editing. Am J Pathol. 2020;190:977–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

