Understanding the epidemiology of iNTS disease in Africa in preparation for future iNTS- vaccine studies in endemic countries: Seroepidemiology in Africa of iNTS (SAiNTS) Study Protocol: Malawi site [Version 9.0]
- PMID: 39183741
- PMCID: PMC11344207
- DOI: 10.12688/wellcomeopenres.18054.2
Understanding the epidemiology of iNTS disease in Africa in preparation for future iNTS- vaccine studies in endemic countries: Seroepidemiology in Africa of iNTS (SAiNTS) Study Protocol: Malawi site [Version 9.0]
Abstract
Background: Non-typhoidal Salmonella (NTS) are a major cause of bloodstream infections amongst children in sub-Saharan Africa. A clear understanding of the seroepidemiology and correlates of protection for invasive NTS (iNTS) in relation to key risk factors (malaria, anaemia, malnutrition) in children in Africa is needed to inform strategies for disease control including vaccine implementation.
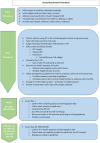
Methodology: The SAiNTS study is a prospective community cohort study with paired serology samples from 2500 Malawian children 0-5 years at baseline and three months to measure age-stratified acquisition of lipopolysaccharide (LPS) O-antigen antibody (IgG) and serum bactericidal activity to the main serovars causing iNTS ( Salmonella Typhimurium and S. Enteritidis). Children are selected from mapped and censused randomly selected households in Chikwawa, Malawi; an area with substantial malaria burden. The sampling framework is set within a malaria vaccination (RTS,S/ AS01) phase 4 cluster randomized trial, known as the Epidemiology Study of Malaria Transmission Intensity (EPI-MAL), allowing exploration of the impact of malaria vaccination on acquisition of immunity to NTS. Risk factor data for invasive disease will be collected using rapid diagnostic tests for malaria and anaemia, anthropometry for malnutrition, and a validated questionnaire for indicators of socioeconomic status, water and sanitation. All data will be recorded through electronic case report forms using the REDCap and the Open Data Kit (ODK) platforms. Stool sample analysis includes NTS culture and pan-Salmonella polymerase chain reaction to assess enteric exposure and biomarkers of environmental enteric dysfunction. Cases with iNTS disease will be followed up for comparison with community controls.
Conclusions: The final cohort of 2500 children will allow investigation into the impact of risk factors for iNTS on the acquisition of immunity in children 0-5 years in an endemic setting, including comparisons to partner seroepidemiology studies in three other sub-Saharan African sites (1000 children per site). The data generated will be key to informing iNTS disease control measures including targeted risk factor interventions and vaccine implementation through investigation of correlates of protection and identifying windows of immune susceptibility in at-risk populations.
Keywords: iNTS; immunoepidemiology; susceptibility; malaria; children; non-typhoidal salmonella.
Copyright: © 2024 Dale H et al.
Conflict of interest statement
No competing interests were disclosed.
Figures




References
-
- GBD 2017 Risk Factor Collaborators: Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1923–94. 10.1016/S0140-6736(18)32225-6 - DOI - PMC - PubMed
-
- International Vaccine Institute: IVI to accelerate efforts in iNTS vaccine development. October 5, 2022. Reference Source
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

