Cholecystokinin receptor type A are involved in the circadian rhythm of the mouse retina
- PMID: 39183886
- PMCID: PMC11341299
- DOI: 10.1016/j.heliyon.2024.e32653
Cholecystokinin receptor type A are involved in the circadian rhythm of the mouse retina
Abstract
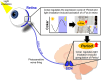
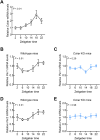
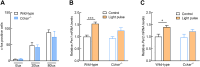
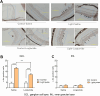
The retina is the only organ projecting external light to the suprachiasmatic nucleus. Cholecystokinin receptor type A (Cckar/Cckar) is one of the essential factors for light reception in retinal cells. As there was a lack of literature on the matter, we aimed to elucidate the cause of the time-dependent phase change in clock gene expression. We found that Cckar mRNA expression in retinal cells exhibited diurnal variations. The rhythm of expression of the clock gene Per1/Per2 in retinal cells was altered in Cckar -/- mice. The light sensitivity of retinal cells was evaluated in wild-type mice, which showed c-Fos was activated in the ganglion cell layer more than in the inner granular layer. This increase in the number of c-Fos-positive cells was suppressed by lorglumide, a Cckar antagonist. Treatment of rat retina primary cells with lorglumide suppressed Per2 transcription, which was altered in a time-dependent manner relative to the Per2 expression. Light irradiation studies in Cckar -/- mice did not exhibit an increase in Period expression in the suprachiasmatic nucleus. These results indicate that Cckar is among the factors that regulate the cycle of clock genes on the retina. Cckar knockout attenuates the light responsiveness of suprachiasmatic nucleus and reduces the expression amplitude of Period genes in the retina. Thus, Cckar may contribute to entrainment of the light environment and maintenance of the expression cycle of Period gene, which is one of the core clock genes.
© 2024 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Expression and light sensitivity of clock genes Per1 and Per2 and immediate-early gene c-fos within the retina of early postnatal Wistar rats.J Comp Neurol. 2010 Sep 1;518(17):3630-44. doi: 10.1002/cne.22421. J Comp Neurol. 2010. PMID: 20589906
-
Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators.PLoS One. 2012;7(6):e38985. doi: 10.1371/journal.pone.0038985. Epub 2012 Jun 11. PLoS One. 2012. PMID: 22701739 Free PMC article.
-
Diabetic retinopathy alters light-induced clock gene expression and dopamine levels in the mouse retina.Mol Vis. 2016 Aug 5;22:959-69. eCollection 2016. Mol Vis. 2016. PMID: 27559292 Free PMC article.
-
Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal Arvicanthis ansorgei.Neuroscience. 2003;116(2):583-91. doi: 10.1016/s0306-4522(02)00654-1. Neuroscience. 2003. PMID: 12559113
-
The Retina and Other Light-sensitive Ocular Clocks.J Biol Rhythms. 2016 Jun;31(3):223-43. doi: 10.1177/0748730416642657. Epub 2016 Apr 19. J Biol Rhythms. 2016. PMID: 27095816 Free PMC article. Review.
References
-
- Moore R.Y., Eichler V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. - PubMed
-
- Moore R.Y., Speh J.C., Card J.P. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J. Comp. Neurol. 1995;352:351–366. - PubMed
-
- Liou S.Y., Shibata S., Iwasaki K., Ueki S. Optic nerve stimulation-induced increase of release of 3H-glutamate and 3H-aspartate but not 3H-GABA from the suprachiasmatic nucleus in slices of rat hypothalamus. Brain Res. Bull. 1986;16(4):527–531. - PubMed
-
- de Vries M.J., Treep J.A., de Pauw E.S., Meijer J.H. The effects of electrical stimulation of the optic nerves and anterior optic chiasm on the circadian activity rhythm of the Syrian hamster: involvement of excitatory amino acids. Brain Res. 1994;642(1–2):206–212. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

