The merger of electro-reduction and hydrogen bonding activation for a radical Smiles rearrangement
- PMID: 39183920
- PMCID: PMC11339951
- DOI: 10.1039/d4sc02821j
The merger of electro-reduction and hydrogen bonding activation for a radical Smiles rearrangement
Abstract
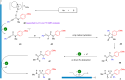
The reductive activation of chemical bonds at less negative potentials provides a foundation for high functional group tolerance and selectivity, and it is one of the central topics in organic electrosynthesis. Along this line, we report the design of a dual-activation mode by merging electro-reduction with hydrogen bonding activation. As a proof of principle, the reduction potential of N-phenylpropiolamide was shifted positively by 218 mV. Enabled by this strategy, the radical Smiles rearrangement of N-arylpropiolamides without external radical precursors and prefunctionalization steps was accomplished. [DBU][HOAc], a readily accessible ionic liquid, was exploited for the first time both as a hydrogen bonding donor and as a supporting electrolyte.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
-
-
Recent reviews on organic electrosynthesis, see:
- Novaes L. F. T. Liu J.-J. Shen Y.-F. Lu L.-X. Meinhardt J. M. Lin S. Chem. Soc. Rev. 2021;50:7941. doi: 10.1039/D1CS00223F. - DOI - PMC - PubMed
- Lam K. Wheelhouse K. M. P. Org. Process Res. Dev. 2021;25:2579. doi: 10.1021/acs.oprd.1c00434. - DOI
- Tay N. E. S. Lehnherr D. Rovis T. Chem. Rev. 2022;122:2487. doi: 10.1021/acs.chemrev.1c00384. - DOI - PMC - PubMed
- Klein M. Waldvogel S. R. Angew. Chem., Int. Ed. 2022;61:e202204140. doi: 10.1002/anie.202204140. - DOI - PMC - PubMed
- Malapit C. A. Prater M. B. Cabrera-Pardo J. R. Li M. Pham T. D. McFadden T. P. Blank S. Minteer S. D. Chem. Rev. 2022;122:3180. doi: 10.1021/acs.chemrev.1c00614. - DOI - PMC - PubMed
- Liu Y.-W. Li P.-F. Wang Y.-W. Qiu Y.-A. Angew. Chem., Int. Ed. 2023;62:e202306679. doi: 10.1002/anie.202306679. - DOI - PubMed
- Wang Y.-L. Dana S. Long H. Xu Y. Li Y.-J. Kaplaneris N. Ackermann L. Chem. Rev. 2023;123:11269. doi: 10.1021/acs.chemrev.3c00158. - DOI - PMC - PubMed
- Kawamata Y. and Baran P., ChemRxiv, 2023, preprint, 10.26434/chemrxiv-2023-glvvh - DOI
- Francke R. Little R. D. Curr. Opin. Electrochem. 2023;40:101315. doi: 10.1016/j.coelec.2023.101315. - DOI
- Zeng L. Wang J.-X. Wang D.-X. Yi H. Lei A.-W. Angew. Chem., Int. Ed. 2023;62:e202309620. doi: 10.1002/anie.202309620. - DOI - PubMed
- Hilt G. Curr. Opin. Electrochem. 2024;43:101425. doi: 10.1016/j.coelec.2023.101425. - DOI
- Chen N. Wu Z.-J. Xu H.-C. Isr. J. Chem. 2024;64:e202300097. doi: 10.1002/ijch.202300097. - DOI
-
and reviews cited therein.
-
-
- Francke R. Little R. D. Chem. Soc. Rev. 2014;43:2492. doi: 10.1039/C3CS60464K. - DOI - PubMed
- Yan M. Kawamata Y. Baran P. S. Chem. Rev. 2017;117:13230. doi: 10.1021/acs.chemrev.7b00397. - DOI - PMC - PubMed
- Inagi S. Fuchigami T. Curr. Opin. Electrochem. 2017;2:32. doi: 10.1016/j.coelec.2017.02.007. - DOI
- Krueger R. Moeller K. D. J. Org. Chem. 2021;86:15847. doi: 10.1021/acs.joc.1c01609. - DOI - PMC - PubMed
-
- Hardwick T. Ahmed N. ACS Sustain. Chem. Eng. 2021;9:4324. doi: 10.1021/acssuschemeng.0c08434. - DOI
- Huang H. Steiniger K. A. Lambert T. H. J. Am. Chem. Soc. 2022;144:12567. doi: 10.1021/jacs.2c01914. - DOI - PubMed
- Wu S.-Z. Kaur J. Karl T. A. Tian X.-H. Barham J. P. Angew. Chem., Int. Ed. 2022;61:e202107811. doi: 10.1002/anie.202107811. - DOI - PMC - PubMed
- Grimaud L. Lakhdar S. Vitale M. R. Curr. Opin. Electrochem. 2023;40:101307. doi: 10.1016/j.coelec.2023.101307. - DOI
- Lepori M. Schmid S. Barham J. P. Beilstein J. Org. Chem. 2023;19:1055. doi: 10.3762/bjoc.19.81. - DOI - PMC - PubMed
- Edgecomb J. M. Alektiar S. N. Cowper N. G. W. Sowin J. A. Wickens Z. K. J. Am. Chem. Soc. 2023;145:20169. doi: 10.1021/jacs.3c06347. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

