Reversible and size-controlled assembly of reflectin proteins using a charged azobenzene photoswitch
- PMID: 39183923
- PMCID: PMC11339800
- DOI: 10.1039/d4sc03299c
Reversible and size-controlled assembly of reflectin proteins using a charged azobenzene photoswitch
Abstract
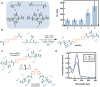
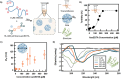
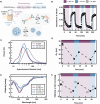
Disordered proteins often undergo a stimuli-responsive, disorder-to-order transition which facilitates dynamic processes that modulate the physiological activities and material properties of cells, such as strength, chemical composition, and reflectance. It remains challenging to gain rapid and spatiotemporal control over such disorder-to-order transitions, which limits the incorporation of these proteins into novel materials. The reflectin protein is a cationic, disordered protein whose assembly is responsible for dynamic color camouflage in cephalopods. Stimuli-responsive control of reflectin's assembly would enable the design of biophotonic materials with tunable color. Herein, a novel, multivalent azobenzene photoswitch is shown to be an effective and non-invasive strategy for co-assembling with reflectin molecules and reversibly controlling assembly size. Photoisomerization between the trans and cis (E and Z) photoisomers promotes or reduces Coulombic interactions, respectively, with reflectin proteins to repeatedly cycle the sizes of the photoswitch-reflectin assemblies between 70 nm and 40 nm. The protein assemblies formed with the trans and cis isomers show differences in interaction stoichiometry and secondary structure, which indicate that photoisomerization modulates the photoswitch-protein interactions to change assembly size. Our results highlight the utility of photoswitchable interactions to control reflectin assembly and provide a tunable synthetic platform that can be adapted to the structure, assembly, and function of other disordered proteins.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Haynes A. Z. Levine M. Anal. Lett. 2021;54:1871–1880. doi: 10.1080/00032719.2020.1828445. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

