Dual inhibitors of Pseudomonas aeruginosa virulence factors LecA and LasB
- PMID: 39183927
- PMCID: PMC11339798
- DOI: 10.1039/d4sc02703e
Dual inhibitors of Pseudomonas aeruginosa virulence factors LecA and LasB
Abstract
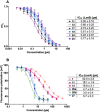
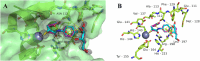
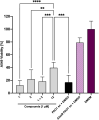
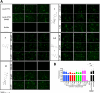
Dual inhibitors of two key virulence factors of Pseudomonas aeruginosa, the lectin LecA and the protease LasB, open up an opportunity in the current antimicrobial-resistance crisis. A molecular hybridization approach enabled the discovery of potent, selective, and non-toxic thiol-based inhibitors, which simultaneously inhibit these two major extracellular virulence factors and therefore synergistically interfere with virulence. We further demonstrated that the dimerization of these monovalent dual inhibitors under physiological conditions affords divalent inhibitors of LecA with a 200-fold increase in binding affinity. The bifunctional LecA/LasB-blocker 12 showed superiority for the inhibition of virulence mediated by both targets over the individual inhibitors or combinations thereof in vitro. Our study sets the stage for a systematic exploration of dual inhibitors as pathoblockers for a more effective treatment of P. aeruginosa infections and the concept can certainly be extended to other targets and pathogens.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Miethke M. Pieroni M. Weber T. Brönstrup M. Hammann P. Halby L. Arimondo P. B. Glaser P. Aigle B. Bode H. B. Moreira R. Li Y. Luzhetskyy A. Medema M. H. Pernodet J.-L. Stadler M. Tormo J. R. Genilloud O. Truman A. W. Weissman K. J. Takano E. Sabatini S. Stegmann E. Brötz-Oesterhelt H. Wohlleben W. Seemann M. Empting M. Hirsch A. K. H. Loretz B. Lehr C.-M. Titz A. Herrmann J. Jaeger T. Alt S. Hesterkamp T. Winterhalter M. Schiefer A. Pfarr K. Hoerauf A. Graz H. Graz M. Lindvall M. Ramurthy S. Karlén A. van Dongen M. Petkovic H. Keller A. Peyrane F. Donadio S. Fraisse L. Piddock L. J. V. Gilbert I. H. Moser H. E. Müller R. Nat. Rev. Chem. 2021;5:726–749. doi: 10.1038/s41570-021-00313-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

