Polydopamine functionalized FeTiO3 nanohexagons for selective and simultaneous electrochemical determination of dopamine and uric acid
- PMID: 39184000
- PMCID: PMC11340443
- DOI: 10.1039/d4ra04148h
Polydopamine functionalized FeTiO3 nanohexagons for selective and simultaneous electrochemical determination of dopamine and uric acid
Abstract
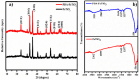
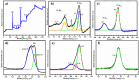
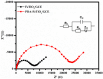
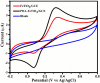
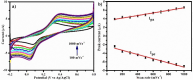
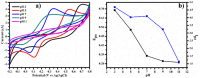
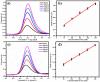
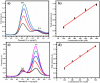
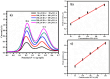
Herein we report the simultaneous detection of dopamine (DA) and uric acid (UA) using polydopamine (PDA) functionalized FeTiO3 nanohexagons. The nanohexagons were hydrothermally synthesized and subsequently functionalized with PDA in a Tris-buffer solution. The PDA functionalized nanostructure was characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and Fourier transform infrared (FTIR), respectively. The SEM and TEM investigations revealed the presence of FeTiO3 nanohexagons along with a peripheral coating of PDA over the nanostructures. The XRD pattern confirmed the formation of the ilmenite structure, while the chemical structure was investigated through XPS and FTIR respectively. Using cyclic voltammetry (CV) the efficacy of FeTiO3-PDA electrode was evaluated toward DA oxidation. The enhanced activity of the functionalized electrode in DA oxidation, as compared to the untreated FeTiO3, may be attributed to the significant presence of hydroxyl, amine, and imine functional groups over the polymer layer. Differential pulse voltammetry (DPV) was utilized for the detection of DA and UA. With a linear range of 50 μM to 250 μM, the detection limits of 0.30 μM and 4.61 μM were determined for DA and UA, respectively. The peak separation of 263 mV between DA and UA demonstrates the sensor's remarkable selectivity. In addition, the study displayed the ability to detect both DA and UA simultaneously, and the validity of the sensor was evaluated in serum samples, respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources

