Dual TTK/PLK1 inhibition has potent anticancer activity in TNBC as monotherapy and in combination
- PMID: 39184047
- PMCID: PMC11341980
- DOI: 10.3389/fonc.2024.1447807
Dual TTK/PLK1 inhibition has potent anticancer activity in TNBC as monotherapy and in combination
Abstract
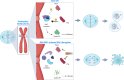
Background: Threonine tyrosine kinase (TTK) and polo-like kinase 1 (PLK1) are common essential kinases that collaborate in activating the spindle assembly checkpoint (SAC) at the kinetochore, ensuring appropriate chromosome alignment and segregation prior to mitotic exit. Targeting of either TTK or PLK1 has been clinically evaluated in cancer patients; however, dual inhibitors have not yet been pursued. Here we present the in vitro and in vivo characterization of a first in class, dual TTK/PLK1 inhibitor (BAL0891).
Methods: Mechanism of action studies utilized biochemical kinase and proteomics-based target-engagement assays. Cellular end-point assays included immunoblot- and flow cytometry-based cell cycle analyses and SAC integrity evaluation using immunoprecipitation and immunofluorescence approaches. Anticancer activity was assessed in vitro using cell growth assays and efficacy was evaluated, alone and in combination with paclitaxel and carboplatin, using mouse models of triple negative breast cancer (TNBC).
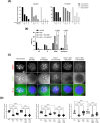
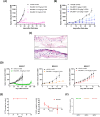
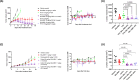
Results: BAL0891 elicits a prolonged effect on TTK, with a transient activity on PLK1. This unique profile potentiates SAC disruption, forcing tumor cells to aberrantly exit mitosis with faster kinetics than observed with a TTK-specific inhibitor. Broad anti-proliferative activity was demonstrated across solid tumor cell lines in vitro. Moreover, intermittent intravenous single-agent BAL0891 treatment of the MDA-MB-231 mouse model of TNBC induced profound tumor regressions associated with prolonged TTK and transient PLK1 in-tumor target occupancy. Furthermore, differential tumor responses across a panel of thirteen TNBC patient-derived xenograft models indicated profound anticancer activity in a subset (~40%). Using a flexible dosing approach, pathologically confirmed cures were observed in combination with paclitaxel, whereas synergy with carboplatin was schedule dependent.
Conclusions: Dual TTK/PLK1 inhibition represents a novel approach for the treatment of human cancer, including TNBC patients, with a potential for potent anticancer activity and a favorable therapeutic index. Moreover, combination approaches may provide an avenue to expand responsive patient populations.
Keywords: BAL0891; Mps-1; PLK1; SAC; TTK; mitotic checkpoint inhibitor.
Copyright © 2024 Zanini, Forster-Gross, Bachmann, Brüngger, McSheehy, Litherland, Burger, Groner, Roceri, Bury, Stieger, Willemsen-Seegers, de Man, Vu-Pham, van Riel, Zaman, Buijsman, Kellenberger and Lane.
Conflict of interest statement
EZ, NF-G, FB, PM, KB, AG, MR, MS and HL were all previous employees of Basilea Pharmaceutica International Ltd, Allschwil. AB, KL, LK and LB are current employees of Basilea Pharmaceutica International Ltd, Allschwil. MS, AB, KL, LK and HL are also Basilea Pharmaceutica International Ltd, Allschwil, stockholders. HL is also a consultant for SillaJen Inc. NW-S, HR and GZ are employees of Oncolines BV and previous Netherlands Translational Research Center NTRC B.V. employees. JM, D-VP and RB are employees of Crossfire Oncology BV formerly Netherlands Translational Research Center NTRC B.V. The studies presented in this manuscript were funded by Basilea Pharmaceutica International Ltd, Allschwil. The funder contributed to study design, as well as the collection, analysis, and interpretation of the results.
Figures

References
-
- Dewhurst SM, McGranahan N, Burrell RA, Rowan AJ, Grönroos E, Endesfelder D, et al. Tolerance of whole- genome doubling propagates chromosomal instability and accelerates cancer genome evolution. Cancer Discovery. (2014) 4:175–85. doi: 10.1158/2159-8290.CD-13-0285 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

