In-utero exposure to tenofovir disoproxil fumarate pre-exposure prophylaxis and growth metrics in HIV unexposed breastfed infants in South Africa: a post hoc analysis of the CAP 016 PrEP in pregnancy RCT
- PMID: 39184858
- PMCID: PMC11341386
- DOI: 10.3389/fped.2024.1447173
In-utero exposure to tenofovir disoproxil fumarate pre-exposure prophylaxis and growth metrics in HIV unexposed breastfed infants in South Africa: a post hoc analysis of the CAP 016 PrEP in pregnancy RCT
Abstract
Objective: We evaluated growth metrics in HIV unexposed African breastfed infants in the first 18 months of life in association with in-utero exposure to Tenofovir Diphosphate Fumarate (TDF) containing pre-exposure prophylaxis (PrEP).
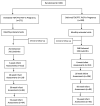
Design: We conducted a secondary data analysis of a TDF-PrEP randomized control trial (CAP016 RCT). Pregnant women without HIV were randomized to initiating TDF-PrEP in pregnancy (Immediate-PrEP-IP) or deferred initiation of TDF-PrEP at cessation of breastfeeding (Deferred-PrEP-DP).
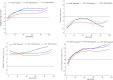
Methods: Infant weight (W), length (L), and head circumference (HC) were measured at birth and 6, 26, 50, and 74 weeks of age. Stored dried blood spot samples from pregnant women randomized to the IP arm were used to measure tenofovir-diphosphate (TFV-DP) levels. Age-stratified mean weight-for-age (WAZ), length-for-age (LAZ), weight-for-length (WLZ), and head circumference-for-age (HCAZ) Z-scores were compared between infants exposed to varying TFV-DP concentrations and infants in the DP arm.
Results: A total of 455 mother-infant pairs were included in the secondary analysis, 228 in the IP arm and 227 in the DP arm. WAZ, LAZ, WLZ, and HCAZ scores were comparable between infants in the Deferred-PrEP arm and Immediate-PrEP arm. In a mixed-effects linear regression model adjusting for maternal age, body mass index, socioeconomic and newborn characteristics, in-utero exposure to varying TFV-DP levels was not associated with WAZ (β = -0.52), LAZ (β = -0.46), WLZ (β = -0.43) and HCAZ (β = -0.11) scores over time.
Conclusion: There was no evidence of an association between growth metrics in the first 18 months of life and in-utero exposure to TFV-DP among breastfed HIV unexposed infants.
Keywords: breastfeeding; exposure; growth; in-utero; preexposure prophylaxis (PrEP).
© 2024 Naidoo, Naidoo, Lombard, Desmond, Clark, Rooney, Gray and Moodley.
Conflict of interest statement
JR and RC are employed by Gilead Sciences. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kinuthia J, Pintye J, Abuna F, Mugwanya KK, Lagat H, Onyango D, et al. PrEP implementation for young women and adolescents (PrIYA) programme. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: results from an implementation programme. Lancet HIV. (2020) 7(1):e38–48. 10.1016/S2352-3018(19)30335-2 - DOI - PMC - PubMed
-
- WHO Technical brief. Preventing HIV During Pregnancy and Breastfeeding in the Context of pre-Exposure Prophylaxis (PrEP). Geneva: World Health Organization; (2017).
LinkOut - more resources
Full Text Sources
Miscellaneous