This is a preprint.
Elevated Lactate in the AML Bone Marrow Microenvironment Polarizes Leukemia-Associated Macrophages via GPR81 Signaling
- PMID: 39185193
- PMCID: PMC11343108
- DOI: 10.1101/2023.11.13.566874
Elevated Lactate in the AML Bone Marrow Microenvironment Polarizes Leukemia-Associated Macrophages via GPR81 Signaling
Abstract
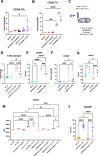
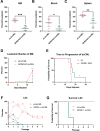
Interactions between acute myeloid leukemia (AML) and the bone marrow microenvironment (BMME) are critical to leukemia progression and chemoresistance. In the solid tumor microenvironment, altered metabolite levels contribute to cancer progression. We performed a metabolomic analysis of AML patient bone marrow serum, revealing increased metabolites compared to age- and sex-matched controls. The most highly elevated metabolite in the AML BMME was lactate. Lactate signaling in solid tumors induces immunosuppressive tumor-associated macrophages and correlates with poor prognosis. This has not yet been studied in the leukemic BMME. Herein, we describe the role of lactate in the polarization of leukemia-associated macrophages (LAMs). Using a murine AML model of blast crisis chronic myelogenous leukemia (bcCML), we characterize the suppressive phenotype of LAMs by surface markers, transcriptomics, and cytokine profiling. Then, mice genetically lacking GPR81, the extracellular lactate receptor, were used to demonstrate GPR81 signaling as a mechanism of both the polarization of LAMs and the direct support of leukemia cells. Furthermore, elevated lactate diminished the function of hematopoietic progenitors and reduced stromal support for normal hematopoiesis. We report microenvironmental lactate as a mechanism of AML-induced immunosuppression and leukemic progression, thus identifying GPR81 signaling as an exciting and novel therapeutic target for treating this devastating disease.
Conflict of interest statement
Conflict of Interest Disclosures Authors have no competing interest to declare.
Figures




References
-
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. The Lancet. 2013;381(9865):484–495. - PubMed
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. New England Journal of Medicine. 2015;373(12):1136–1152. - PubMed
-
- Chang H-Y, Rodriguez V, Narboni G, Bodey GP, Luna MA, Freireich EJ. CAUSES OF DEATH IN ADULTS WITH ACUTE LEUKEMIA. Medicine. 1976;55(3). - PubMed
-
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
