This is a preprint.
Myosin forces elicit an F-actin structural landscape that mediates mechanosensitive protein recognition
- PMID: 39185238
- PMCID: PMC11343212
- DOI: 10.1101/2024.08.15.608188
Myosin forces elicit an F-actin structural landscape that mediates mechanosensitive protein recognition
Abstract
Cells mechanically interface with their surroundings through cytoskeleton-linked adhesions, allowing them to sense physical cues that instruct development and drive diseases such as cancer. Contractile forces generated by myosin motor proteins mediate these mechanical signal transduction processes through unclear protein structural mechanisms. Here, we show that myosin forces elicit structural changes in actin filaments (F-actin) that modulate binding by the mechanosensitive adhesion protein α-catenin. Using correlative cryo-fluorescence microscopy and cryo-electron tomography, we identify F-actin featuring domains of nanoscale oscillating curvature at cytoskeleton-adhesion interfaces enriched in zyxin, a marker of actin-myosin generated traction forces. We next introduce a reconstitution system for visualizing F-actin in the presence of myosin forces with cryo-electron microscopy, which reveals morphologically similar superhelical F-actin spirals. In simulations, transient forces mimicking tugging and release of filaments by motors produce spirals, supporting a mechanistic link to myosin's ATPase mechanochemical cycle. Three-dimensional reconstruction of spirals uncovers extensive asymmetric remodeling of F-actin's helical lattice. This is recognized by α-catenin, which cooperatively binds along individual strands, preferentially engaging interfaces featuring extended inter-subunit distances while simultaneously suppressing rotational deviations to regularize the lattice. Collectively, we find that myosin forces can deform F-actin, generating a conformational landscape that is detected and reciprocally modulated by a mechanosensitive protein, providing a direct structural glimpse at active force transduction through the cytoskeleton.
Keywords: Actin; cryo-electron microscopy; mechanobiology; mechanotransduction; myosin.
Conflict of interest statement
Competing Interests The authors have no competing interests to declare.
Figures

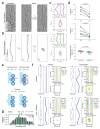

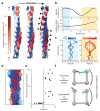
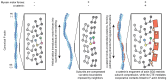
References
-
- Spudich J. A. The myosin swinging cross-bridge model. Nat. Rev. Mol. Cell Biol. 2, 387–392 (2001). - PubMed
-
- Sellers J. R. Myosins: a diverse superfamily. Biochim. Biophys. Acta 1496, 3–22 (2000). - PubMed
-
- Charras G. & Yap A. S. Tensile Forces and Mechanotransduction at Cell-Cell Junctions. Curr. Biol. CB 28, R445–R457 (2018). - PubMed
-
- Ridley A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003). - PubMed
Methods References:
-
- Xu A. & Xu C. FastTomo: A SerialEM Script for Collecting Electron Tomography Data. 2021.03.16.435675 Preprint at 10.1101/2021.03.16.435675 (2021). - DOI
-
- Mastronarde D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
