Selective Chirality-Driven Photopolymerization of Diacetylene Crystals
- PMID: 39185357
- PMCID: PMC11342933
- DOI: 10.1021/acs.cgd.4c00844
Selective Chirality-Driven Photopolymerization of Diacetylene Crystals
Abstract
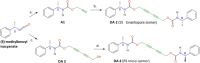
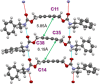
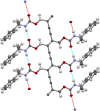
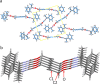
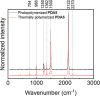
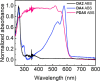
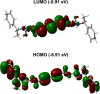
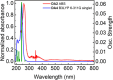
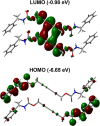
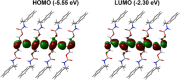
Crystal engineering of two diacetylene monomers was achieved by branching two chiral groups [R = PhC*MeNH(CO2)CH2] exhibiting an enantiopure configuration of S,S-(DA2) and an achiral R,S-meso-isomer (DA4). The X-ray structures of DA2 and DA4 reveal the presence of supramolecular arrangements driven by intermolecular H-bonding. A significant intermolecular closer proximity in DA4 than that in DA2 is depicted, ultimately resulting in a slow thermal (days) and swift (min) photochemical polymerization of DA4 to form PDA5, whereas DA2 is unreactive. DFT computations indicate that in both cases the lowest energy-excited state is the charge-transfer state [CT; PhC*MeNH(CO2) → π*(-C≡C-C≡C-)]. Therefore, this outcome illustrates a drastic selectivity via a settle change in a carbon configuration. Analysis demonstrates that PDA5 is nonemissive and that its coloration arises from a π → π* excitation of the polymer backbone (DFT computations).
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

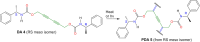

References
-
- Hall A. V.; Musa O. M.; Steed J. W. Properties and Applications of Stimuli-Responsive Diacetylenes. Cryst. Growth Des. 2021, 21 (6), 3614–3638. 10.1021/acs.cgd.1c00300. - DOI
-
- Weston M.; Tjandra A. D.; Chandrawati R. Tuning Chromatic Response, Sensitivity, and Specificity of Polydiacetylene-Based Sensors. Polym. Chem. 2020, 11 (2), 166–183. 10.1039/C9PY00949C. - DOI
-
- Yu Z.; MuYu C.; Xu H.; Zhao J.; Yang G. Recent Progress in the Design of Conjugated Polydiacetylenes with Reversible Thermochromic Performance: A Review. Polym. Chem. 2023, 14 (19), 2266–2290. 10.1039/D3PY00213F. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
