BAFF neutralization impairs the autoantibody-mediated clearance of dead adipocytes and aggravates obesity-induced insulin resistance
- PMID: 39185417
- PMCID: PMC11341376
- DOI: 10.3389/fimmu.2024.1436900
BAFF neutralization impairs the autoantibody-mediated clearance of dead adipocytes and aggravates obesity-induced insulin resistance
Abstract
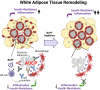
B cell-activating factor (BAFF) is a critical TNF-family cytokine that regulates homeostasis and peripheral tolerance of B2 cells. BAFF overproduction promotes autoantibody generation and autoimmune diseases. During obesity, BAFF is predominantly produced by white adipose tissue (WAT), and IgG autoantibodies against adipocytes are identified in the WAT of obese humans. However, it remains to be determined if the autoantibodies formed during obesity affect WAT remodeling and systemic insulin resistance. Here, we show that IgG autoantibodies are generated in high-fat diet (HFD)-induced obese mice that bind to apoptotic adipocytes and promote their phagocytosis by macrophages. Next, using murine models of obesity in which the gonadal WAT undergoes remodeling, we found that BAFF neutralization depleted IgG autoantibodies, increased the number of dead adipocytes, and exacerbated WAT inflammation and insulin resistance. RNA sequencing of the stromal vascular fraction from the WAT revealed decreased expression of immunoglobulin light-chain and heavy-chain variable genes suggesting a decreased repertoire of B cells after BAFF neutralization. Further, the B cell activation and the phagocytosis pathways were impaired in the WAT of BAFF-neutralized mice. In vitro, plasma IgG fractions from BAFF-neutralized mice reduced the phagocytic clearance of apoptotic adipocytes. Altogether, our study suggests that IgG autoantibodies developed during obesity, at least in part, dampens exacerbated WAT inflammation and systemic insulin resistance.
Keywords: B2 cell; BAFF; IgG autoantibodies; inflammation; obesity-induced insulin resistance; white adipose tissue.
Copyright © 2024 Lempicki, Gray, Abuna, Murata, Divanovic, McNamara and Meher.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

