TolC facilitates the intracellular survival and immunomodulation of Salmonella Typhi in human host cells
- PMID: 39185619
- PMCID: PMC11385165
- DOI: 10.1080/21505594.2024.2395831
TolC facilitates the intracellular survival and immunomodulation of Salmonella Typhi in human host cells
Abstract
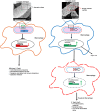
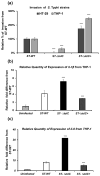
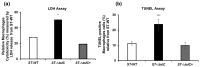
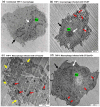
Salmonella enterica serovar Typhi (S. Typhi) causes typhoid fever, a systemic infection that affects millions of people worldwide. S. Typhi can invade and survive within host cells, such as intestinal epithelial cells and macrophages, by modulating their immune responses. However, the immunomodulatory capability of S. Typhi in relation to TolC-facilitated efflux pump function remains unclear. The role of TolC, an outer membrane protein that facilitates efflux pump function, in the invasion and immunomodulation of S. Typhi, was studied in human intestinal epithelial cells and macrophages. The tolC deletion mutant of S. Typhi was compared with the wild-type and its complemented strain in terms of their ability to invade epithelial cells, survive and induce cytotoxicity in macrophages, and elicit proinflammatory cytokine production in macrophages. The tolC mutant, which has a defective outer membrane, was impaired in invading epithelial cells compared to the wild-type strain, but the intracellular presence of the tolC mutant exhibited greater cytotoxicity and induced higher levels of proinflammatory cytokines (IL-1β and IL-8) in macrophages compared to the wild-type strain. These effects were reversed by complementing the tolC mutant with a functional tolC gene. Our results suggest that TolC plays a role in S. Typhi to efficiently invade epithelial cells and suppress host immune responses during infection. TolC may be a potential target for the development of novel therapeutics against typhoid fever.
Keywords: Salmonella Typhi; immunomodulation of macrophages and epithelial cells; immunomodulatory ability of pathogens; invasion of bacteria into host cells; multidrug efflux pump AcrAB-TolC; mutant induce cytotoxic proinflammatory responses in host cell.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
