Clinical phenotyping uncovers heterogeneous associations between corticosteroid treatment and survival in critically ill COVID-19 patients
- PMID: 39186112
- PMCID: PMC11541258
- DOI: 10.1007/s00134-024-07593-3
Clinical phenotyping uncovers heterogeneous associations between corticosteroid treatment and survival in critically ill COVID-19 patients
Abstract
Purpose: Disease heterogeneity in coronavirus disease 2019 (COVID-19) may render the current one-size-fits-all treatment approach suboptimal. We aimed to identify and immunologically characterize clinical phenotypes among critically ill COVID-19 patients, and to assess heterogeneity of corticosteroid treatment effect.
Methods: We applied consensus k-means clustering on 21 clinical parameters obtained within 24 h after admission to the intensive care unit (ICU) from 13,279 COVID-19 patients admitted to 82 Dutch ICUs from February 2020 to February 2022. Derived phenotypes were reproduced in 6225 COVID-19 ICU patients from Spain (February 2020 to December 2021). Longitudinal immunological characterization was performed in three COVID-19 ICU cohorts from the Netherlands and Germany, and associations between corticosteroid treatment and survival were assessed across phenotypes.
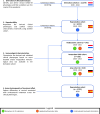
Results: We derived three phenotypes: COVIDICU1 (43% of patients) consisted of younger patients with the lowest Acute Physiology And Chronic Health Evaluation (APACHE) scores, highest body mass index (BMI), lowest PaO2/FiO2 ratio, and a 90-day in-hospital mortality rate of 18%. COVIDICU2 patients (37%) had the lowest BMI, were older and had higher APACHE scores and mortality rate (24%) than COVIDICU1. Patients with COVIDICU3 (20%) were the eldest with the most comorbidities, the highest APACHE scores, acute kidney injury and metabolic dysregulations, and the highest mortality rate (47%). These patients also displayed the most pronounced inflammatory response. Corticosteroid therapy started at day 5 [2-9] after ICU admission and administered for 5 [3-7] days was associated with an increased risk for 90-day mortality in patients with the COVIDICU1 and COVIDICU2 phenotypes (hazard ratio [HR] 1.59 [1.09-2.31], p = 0.015 and HR 1.79 [1.42-2.26], p < 0.001, respectively), but not in patients with the COVIDICU3 phenotype (HR 1.08 [0.76-1.54], p = 0.654).
Conclusion: Our multinational study identified three distinct clinical COVID-19 phenotypes, each exhibiting marked differences in demographic, clinical, and immunological features, and in the response to late and short-term corticosteroid treatment.
Keywords: COVID-19; Corticosteroids; Heterogeneity; Inflammation; Machine learning; Phenotypes.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ (2021) Dexamethasone in hospitalized patients with COVID-19. N Engl J Med 384:693–704 - DOI - PMC - PubMed
-
- Group WHOREAfC-TW, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Juni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Moller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC (2020) Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 324:1330–1341 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

