Agonism of β3-Adrenoceptors Inhibits Pathological Retinal Angiogenesis in the Model of Oxygen-Induced Retinopathy
- PMID: 39186263
- PMCID: PMC11361380
- DOI: 10.1167/iovs.65.10.34
Agonism of β3-Adrenoceptors Inhibits Pathological Retinal Angiogenesis in the Model of Oxygen-Induced Retinopathy
Retraction in
-
Retraction of: Agonism of β3-Adrenoceptors Inhibits Pathological Retinal Angiogenesis in the Model of Oxygen-Induced Retinopathy.Invest Ophthalmol Vis Sci. 2025 Nov 3;66(14):16. doi: 10.1167/iovs.66.14.16. Invest Ophthalmol Vis Sci. 2025. PMID: 41201294 Free PMC article. No abstract available.
Abstract
Purpose: In response to hypoxia, sympathetic fibers to the retina activate β-adrenoceptors (β-ARs) that play an important role in the regulation of vascular and neuronal functions. We investigated the role of β3-AR using the mouse model of oxygen-induced retinopathy (OIR).
Methods: Mouse pups were exposed to 75% oxygen at postnatal day 7 (PD7) followed by a return to room air at PD12. The β3-AR preferential agonist BRL37344 was subcutaneously administered once daily at different times after the return to room air. At PD17, the OIR mice underwent flash and pattern electroretinogram. After sacrifice, retinal wholemounts were used for vessel staining or immunohistochemistry for astrocytes, Müller cells, or retinal ganglion cells (RGCs). In retinal homogenates, the levels of markers associated with neovascularization (NV), the blood-retinal barrier (BRB), or astrocytes were determined by western blot, and quantitative reverse-transcription polymerase chain reaction was used to assess β3-AR messenger. Administration of the β3-AR antagonist SR59230A was performed to verify BRL37344 selectivity.
Results: β3-AR expression is upregulated in response to hypoxia, but its increase is prevented by BRL37344, which counteracts NV by inhibiting the pro-angiogenic pathway, activating the anti-angiogenic pathway, recovering BRB-associated markers, triggering nitric oxide production, and favoring revascularization of the central retina through recovered density of astrocytes that ultimately counteracts NV in the midperiphery. Vasculature rescue prevents dysfunctional retinal activity and counteracts OIR-associated retinal ganglion cell loss.
Conclusions: β3-AR has emerged as a crucial intermediary in hypoxia-dependent NV, suggesting a role of β3-AR agonists in the treatment of proliferative retinopathies.
Conflict of interest statement
Disclosure:
Figures
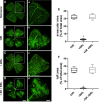
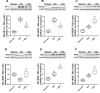

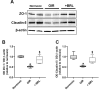
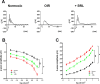
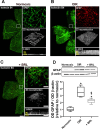
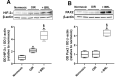



References
-
- Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P.. The β-adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Prog Retin Eye Res . 2014; 42: 103–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

