Long-Term Outcomes of Adjuvant Trastuzumab for 9 Weeks or 1 Year for ERBB2-Positive Breast Cancer: A Secondary Analysis of the SOLD Randomized Clinical Trial
- PMID: 39186271
- PMCID: PMC12507463
- DOI: 10.1001/jamanetworkopen.2024.29772
Long-Term Outcomes of Adjuvant Trastuzumab for 9 Weeks or 1 Year for ERBB2-Positive Breast Cancer: A Secondary Analysis of the SOLD Randomized Clinical Trial
Abstract
Importance: The standard adjuvant treatment for patients with ERRB2-positive breast cancer is chemotherapy plus 1 year of trastuzumab. Shorter durations of trastuzumab administration improve cardiac safety, but more information is needed about their effect on survival.
Objective: To compare survival outcomes after 9-week vs 1-year administration of trastuzumab with the same adjuvant chemotherapy.
Design, setting, and participants: This post hoc secondary analysis of an open-label, multicenter, noninferiority-design randomized clinical trial included women aged 18 years or older with early ERBB2-positive, axillary node-negative or axillary node-positive breast cancer who were enrolled from January 3, 2008, to December 16, 2014, at 65 centers in 7 European countries. The current exploratory analysis was conducted after achieving the maximum attainable follow-up data when the last patient enrolled had completed the last scheduled visit in December 2022.
Intervention: Chemotherapy consisted of 3 cycles of docetaxel administered at 3-week intervals followed by 3 cycles of fluorouracil, epirubicin, and cyclophosphamide at 3-week intervals. Trastuzumab was administered in both groups for 9 weeks concomitantly with docetaxel. In the 9-week group, no further trastuzumab was administered after chemotherapy, whereas in the 1-year group, trastuzumab was continued after chemotherapy to complete 1 year of administration.
Main outcomes and measures: The primary objective was disease-free survival (DFS). Distant DFS and OS were secondary objectives. Survival between groups was compared using the Kaplan-Meier method and log-rank test or univariable Cox proportional hazards regression.
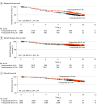
Results: Among the 2174 women analyzed, median age was 56 years (IQR, 48-64 years). The median follow-up time was 8.1 years (IQR, 8.0-8.9 years); 357 DFS events and 176 deaths occurred. Trastuzumab for 9 weeks was associated with shorter DFS compared with trastuzumab for 1 year (hazard ratio [HR], 1.36; 90% CI, 1.14-1.62); 10-year DFS was 80.3% in the 1-year group vs 78.6% in the 9-week group. The 5-year and 10-year OS rates were comparable between the 9-week and 1-year groups (95.0% vs 95.9% and 89.1% vs 88.2%, respectively; HR for all time points, 1.20; 90% CI, 0.94-1.54). In multivariable analyses, 9-week treatment was associated with shorter DFS compared with 1-year treatment (HR for recurrence or death, 1.36; 95% CI, 1.10-1.68; P = .005), but there was no between-group difference in OS (HR, 1.22; 95% CI, 0.90-1.64; P = .20). Only 4 patients (0.2%) died of a cardiac cause.
Conclusions and relevance: In this secondary analysis of a randomized clinical trial, 1-year vs 9-week adjuvant trastuzumab was associated with improved DFS among patients with ERRB2-positive breast cancer receiving chemotherapy, but there was no significant difference in OS between the groups.
Trial registration: ClinicalTrials.gov Identifier: NCT00593697.
Conflict of interest statement
Figures
References
-
- Park YH, Senkus-Konefka E, Im SA, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with early breast cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31(4):451-469. doi: 10.1016/j.annonc.2020.01.008 - DOI - PubMed
-
- Mavroudis D, Saloustros E, Malamos N, et al. ; Breast Cancer Investigators of Hellenic Oncology Research Group (HORG), Athens, Greece . Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26(7):1333-1340. doi: 10.1093/annonc/mdv213 - DOI - PubMed
-
- Pivot X, Romieu G, Debled M, et al. ; PHARE trial investigators . 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet. 2019;393(10191):2591-2598. doi: 10.1016/S0140-6736(19)30653-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous