Endovascular vs Medical Management of Acute Basilar Artery Occlusion: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 39186280
- PMCID: PMC11348088
- DOI: 10.1001/jamaneurol.2024.2652
Endovascular vs Medical Management of Acute Basilar Artery Occlusion: A Secondary Analysis of a Randomized Clinical Trial
Erratum in
-
Error in Figure.JAMA Neurol. 2024 Oct 1;81(10):1103. doi: 10.1001/jamaneurol.2024.3566. JAMA Neurol. 2024. PMID: 39401027 Free PMC article. No abstract available.
Abstract
Importance: In several randomized clinical trials, endovascular thrombectomy led to better functional outcomes than conventional treatment at 90 days poststroke in patients with acute basilar artery occlusion. However, the long-term clinical outcomes of these patients have not been well delineated.
Objective: To evaluate 1-year clinical outcomes in patients with acute basilar artery occlusion following endovascular thrombectomy vs control.
Design, setting, and participants: This study is an extension of the ATTENTION trial, a multicenter, randomized clinical trial. Patients were included between February 2021 and January 2022, with 1-year follow-up through April 2023. This multicenter, population-based study was conducted at 36 comprehensive stroke sites. Patients with acute basilar artery occlusion within 12 hours of estimated symptom onset were included. Of the 342 patients randomized in the ATTENTION trial, 330 (96.5%) had 1-year follow-up information available.
Exposures: Endovascular thrombectomy (thrombectomy group) vs best medical treatment (control group).
Main outcomes and measures: The primary outcome was defined as a score of 0 to 3 on the modified Rankin Scale (mRS) at 1 year. Secondary outcomes were functional independence (mRS score 0-2), excellent outcome (mRS score 0-1), level of disability (distribution of all 7 mRS scores), mortality, and health-related quality of life at 1 year.
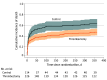
Results: Among 330 patients who had 1-year follow-up data, 227 (68.8%) were male, and the mean (SD) age was 67.0 (10.7) years. An mRS score 0 to 3 at 1 year was achieved by 99 of 222 patients (44.6%) in the thrombectomy group and 21 of 108 (19.4%) in the control group (adjusted rate ratio, 2.23; 95% CI, 1.51-3.29). Mortality at 1 year compared with 90 days was more frequent in both the thrombectomy group (101 of 222 [45.5%] vs 83 of 226 [36.7%]) and the control group (69 of 108 [63.9%] vs 63 of 114 [55.3%]). Excellent outcome (mRS score 0-1) at 1 year compared with 90 days increased in the thrombectomy group (62 of 222 [27.9%] vs 45 of 226 [19.9%]) but not in the control group (9 of 108 [8.3%] vs 9 of 114 [7.9%]) resulting in a magnified treatment benefit.
Conclusions and relevance: Among patients with basilar artery occlusion within 12 hours of onset, the benefits of endovascular thrombectomy at 1 year compared with 90 days were sustained for favorable (mRS score 0-3) outcome and enhanced for excellent (mRS score 0-1) outcome.
Conflict of interest statement
Figures


References
-
- Abdalkader M, Finitsis S, Li C, et al. ; BasilaR Artery Ischemia Network STudy of Endovascular versus Medical Management (BRAINSTEM) Group . Endovascular versus medical management of acute basilar artery occlusion: a systematic review and meta-analysis of the randomized controlled trials. J Stroke. 2023;25(1):81-91. doi: 10.5853/jos.2022.03755 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

