SC134-TCB Targeting Fucosyl-GM1, a T Cell-Engaging Antibody with Potent Antitumor Activity in Preclinical Small Cell Lung Cancer Models
- PMID: 39186309
- PMCID: PMC11532774
- DOI: 10.1158/1535-7163.MCT-24-0187
SC134-TCB Targeting Fucosyl-GM1, a T Cell-Engaging Antibody with Potent Antitumor Activity in Preclinical Small Cell Lung Cancer Models
Abstract
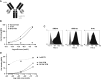
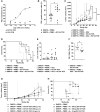
Small cell lung cancer (SCLC) is an aggressive disease with limited treatment options. Fucosyl-GM1 (FucGM1) is a glycolipid overexpressed in the majority of SCLC tumors but virtually absent from normal healthy tissues. In this study, we validate a FucGM1-targeting T cell-redirecting bispecific (TCB) antibody for the treatment of SCLC. More than 80% of patient-derived xenograft tissues of SCLC expressed FucGM1, whereas only three normal human tissues: pituitary, thymus, and skin expressed low and focal FucGM1. A FucGM1-targeting TCB (SC134-TCB), based on the Fc-silenced humanized SC134 antibody, exhibited nanomolar efficiency in FucGM1 glycolipid and SCLC cell surface binding. SC134-TCB showed potent ex vivo killing of SCLC cell lines with donor-dependent EC50 ranging from 7.2 pmol/L up to 211.0 pmol/L, effectively activating T cells, with picomolar efficiency, coinciding with target-dependent cytokine production such as IFNγ, IL2, and TNFα and robust proliferation of both CD4 and CD8 T cells. The ex vivo SC134-TCB tumor controlling activity translated into an effective in vivo anti-DMS79 tumor therapy, resulting in 100% tumor-free survival in a human peripheral blood mononuclear cell admixed setting and 40% overall survival (55% tumor growth inhibition) with systemically administered human peripheral blood mononuclear cells. Combination treatment with atezolizumab further enhanced survival and tumor growth inhibition (up to 73%). A 10-fold SC134-TCB dose reduction maintained the strong in vivo antitumor impact, translating into 70% overall survival (P < 0.0001). Whole-blood incubation with SC134-TCB, as well as healthy human primary cells analysis, revealed no target-independent cytokine production. SC134-TCB presents an attractive candidate to deliver an effective immunotherapy treatment option for patients with SCLC.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
All authors except Z. Dunster report employment with Scancell. L. Durrant reports other support from Scancell during the conduct of the study, as well as a patent for WO2021/043810A1 pending to Scancell. No disclosures were reported by Z. Dunster.
Figures




References
-
- Chari A, Minnema MC, Berdeja JG, Oriol A, van de Donk NWCJ, Rodríguez-Otero P, et al. . Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med 2022;387:2232–44. - PubMed
-
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 1985;314:53–7. - PubMed
-
- Drivsholm L, Vangsted A, Pallesen T, Hansen M, Dombernowsky P, Hirsch F, et al. . Fucosyl-GM1 in small-cell lung cancer. A comparison with the tumour marker neuron-specific enolase. Ann Oncol 1994;5:623–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

