Creating a Modified Version of the Cambridge Multimorbidity Score to Predict Mortality in People Older Than 16 Years: Model Development and Validation
- PMID: 39186368
- PMCID: PMC11384182
- DOI: 10.2196/56042
Creating a Modified Version of the Cambridge Multimorbidity Score to Predict Mortality in People Older Than 16 Years: Model Development and Validation
Abstract
Background: No single multimorbidity measure is validated for use in NHS (National Health Service) England's General Practice Extraction Service Data for Pandemic Planning and Research (GDPPR), the nationwide primary care data set created for COVID-19 pandemic research. The Cambridge Multimorbidity Score (CMMS) is a validated tool for predicting mortality risk, with 37 conditions defined by Read Codes. The GDPPR uses the more internationally used Systematized Nomenclature of Medicine clinical terms (SNOMED CT). We previously developed a modified version of the CMMS using SNOMED CT, but the number of terms for the GDPPR data set is limited making it impossible to use this version.
Objective: We aimed to develop and validate a modified version of CMMS using the clinical terms available for the GDPPR.
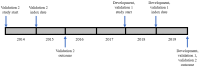
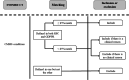
Methods: We used pseudonymized data from the Oxford-Royal College of General Practitioners Research and Surveillance Centre (RSC), which has an extensive SNOMED CT list. From the 37 conditions in the original CMMS model, we selected conditions either with (1) high prevalence ratio (≥85%), calculated as the prevalence in the RSC data set but using the GDPPR set of SNOMED CT codes, divided by the prevalence included in the RSC SNOMED CT codes or (2) conditions with lower prevalence ratios but with high predictive value. The resulting set of conditions was included in Cox proportional hazard models to determine the 1-year mortality risk in a development data set (n=500,000) and construct a new CMMS model, following the methods for the original CMMS study, with variable reduction and parsimony, achieved by backward elimination and the Akaike information stopping criterion. Model validation involved obtaining 1-year mortality estimates for a synchronous data set (n=250,000) and 1-year and 5-year mortality estimates for an asynchronous data set (n=250,000). We compared the performance with that of the original CMMS and the modified CMMS that we previously developed using RSC data.
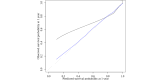
Results: The initial model contained 22 conditions and our final model included 17 conditions. The conditions overlapped with those of the modified CMMS using the more extensive SNOMED CT list. For 1-year mortality, discrimination was high in both the derivation and validation data sets (Harrell C=0.92) and 5-year mortality was slightly lower (Harrell C=0.90). Calibration was reasonable following an adjustment for overfitting. The performance was similar to that of both the original and previous modified CMMS models.
Conclusions: The new modified version of the CMMS can be used on the GDPPR, a nationwide primary care data set of 54 million people, to enable adjustment for multimorbidity in predicting mortality in people in real-world vaccine effectiveness, pandemic planning, and other research studies. It requires 17 variables to produce a comparable performance with our previous modification of CMMS to enable it to be used in routine data using SNOMED CT.
Keywords: COVID-19; calibration; computerized medical records; discrimination; multimorbidity; pandemics; predictive model; prevalence; systematized nomenclature of medicine; systems.
©Debasish Kar, Kathryn S Taylor, Mark Joy, Sudhir Venkatesan, Wilhelmine Meeraus, Sylvia Taylor, Sneha N Anand, Filipa Ferreira, Gavin Jamie, Xuejuan Fan, Simon de Lusignan. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 26.08.2024.
Conflict of interest statement
Conflicts of Interest: SdL is the principal investigator for real-world effectiveness of the Oxford-AstraZeneca COVID-19 vaccine in England (RAVEN; EUPAS43571) funded by AstraZeneca. SdL is also the Director of the Oxford-Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC), which is included in his academic role at the University of Oxford. He has received research funding through his University from AstraZeneca, GlaxoSmithKline (GSK), Lily, Moderna, Medical Science Division (MSD), Sanofi, Seqirus, and Takeda. He has also served as an advisory board member for AstraZeneca, GSK, Sanofi, Seqirus, and Pfizer. SV, WM and ST are employees of AstraZeneca and may own stock/shares. ST reports ownership of GSK stocks/shares.
Figures
References
-
- Aubert CE, Schnipper JL, Roumet M, Marques-Vidal P, Stirnemann J, Auerbach AD, Zimlichman E, Kripalani S, Vasilevskis EE, Robinson E, Fletcher GS, Aujesky D, Limacher A, Donzé J. Best definitions of multimorbidity to identify patients with high health care resource utilization. Mayo Clin Proc Innov Qual Outcomes. 2020;4(1):40–49. doi: 10.1016/j.mayocpiqo.2019.09.002. https://boris.unibe.ch/id/eprint/140685 S2542-4548(19)30144-4 - DOI - PMC - PubMed
-
- Dambha-Miller H, Simpson G, Hobson L, Roderick P, Little P, Everitt H, Santer M. Integrated primary care and social services for older adults with multimorbidity in England: a scoping review. BMC Geriatr. 2021;21(1):674. doi: 10.1186/s12877-021-02618-8. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-021-02618-8 10.1186/s12877-021-02618-8 - DOI - PMC - PubMed
-
- Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK biobank participants. Lancet Public Health. 2018;3(7):e323–e332. doi: 10.1016/S2468-2667(18)30091-4. https://linkinghub.elsevier.com/retrieve/pii/S2468-2667(18)30091-4 S2468-2667(18)30091-4 - DOI - PMC - PubMed
-
- Smith SM, Wallace E, O'Dowd T, Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3(3):CD006560. doi: 10.1002/14651858.CD006560.pub3. https://europepmc.org/abstract/MED/26976529 - DOI - PMC - PubMed
-
- Pearson-Stuttard J, Ezzati M, Gregg EW. Multimorbidity-a defining challenge for health systems. Lancet Public Health. 2019;4(12):e599–e600. doi: 10.1016/S2468-2667(19)30222-1. https://linkinghub.elsevier.com/retrieve/pii/S2468-2667(19)30222-1 S2468-2667(19)30222-1 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

