Bimolecular Sandwich Aggregates of Porphyrin Nanorings
- PMID: 39186461
- PMCID: PMC11403599
- DOI: 10.1021/jacs.4c09267
Bimolecular Sandwich Aggregates of Porphyrin Nanorings
Abstract
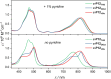
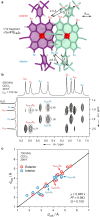
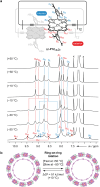
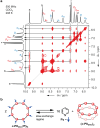
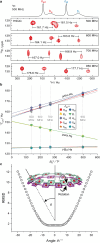
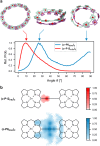
Extended π-systems often form supramolecular aggregates, drastically changing their optical and electronic properties. However, aggregation processes can be difficult to characterize or predict. Here, we show that butadiyne-linked 8- and 12-porphyrin nanorings form stable and well-defined bimolecular aggregates with remarkably sharp NMR spectra, despite their dynamic structures and high molecular weights (12.7 to 26.0 kDa). Pyridine breaks up the aggregates into their constituent rings, which are in slow exchange with the aggregates on the NMR time scale. All the aggregates have the same general two-layer sandwich structure, as deduced from NMR spectroscopy experiments, including 1H DOSY, 1H-1H COSY, TOCSY, NOESY, and 1H-13C HSQC. This structure was confirmed by analysis of residual dipolar couplings from 13C-coupled 1H-13C HSQC experiments on one of the 12-ring aggregates. Variable-temperature NMR spectroscopy revealed an internal ring-on-ring rotation process by which two π-π stacked conformers interconvert via a staggered conformation. A slower dynamic process, involving rotation of individual porphyrin units, was also detected by exchange spectroscopy in the 8-ring aggregates, implying partial disaggregation and reassociation. Molecular dynamics simulations indicate that the 8-ring aggregates are bowl-shaped and highly fluxional, compared to the 12-ring aggregates, which are cylindrical. This work demonstrates that large π-systems can form surprisingly well-defined aggregates and may inspire the design of other noncovalent assemblies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

