Age-Associated Contraction of Tumor-Specific T Cells Impairs Antitumor Immunity
- PMID: 39186561
- PMCID: PMC11532741
- DOI: 10.1158/2326-6066.CIR-24-0463
Age-Associated Contraction of Tumor-Specific T Cells Impairs Antitumor Immunity
Abstract
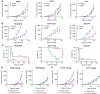
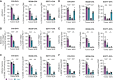
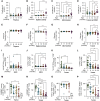
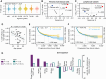
Progressive decline of the adaptive immune system with increasing age coincides with a sharp increase in cancer incidence. In this study, we set out to understand whether deficits in antitumor immunity with advanced age promote tumor progression and/or drive resistance to immunotherapy. We found that multiple syngeneic cancers grew more rapidly in aged versus young adult mice, driven by dysfunctional CD8+ T-cell responses. By systematically mapping immune cell profiles within tumors, we identified loss of tumor antigen-specific CD8+ T cells as a primary feature accelerating the growth of tumors in aged mice and driving resistance to immunotherapy. When antigen-specific T cells from young adult mice were administered to aged mice, tumor outgrowth was delayed and the aged animals became sensitive to PD-1 blockade. These studies reveal how age-associated CD8+ T-cell dysfunction may license tumorigenesis in elderly patients and have important implications for the use of aged mice as preclinical models of aging and cancer.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
P. Georgiev reports personal fees from RA Capitall and Astro Therapeutics outside the submitted work. S. Han reports personal fees from Merck KGaA outside the submitted work. J.M. Drijvers reports personal fees from Abata Therapeutics outside the submitted work. B.C. Miller reports personal fees from Rheos Medicines, Cellarity, Lifeomic, and Telix Pharmaceuticals outside the submitted work. G.J. Freeman reports grants from National Institutes of Health during the conduct of the study; personal fees from iTeos, NextPoint, IgM, GV20, IOME, Bioentre, Santa Ana Bio, Simcere of America, and Geode outside the submitted work; in addition, G.J. Freeman has a patent for PD-L1/PD-1 pathway issued, licensed, and with royalties paid from Bristol-Myers Squibb, a patent for PD-L1/PD-1 pathway issued, licensed, and with royalties paid from Roche, a patent for PD-L1/PD-1 pathway issued, licensed, and with royalties paid from Eli Lilly, and a patent for PD-L1/PD-1 pathway issued, licensed, and with royalties paid from Novartis; and equity in Nextpoint, Triursus, Xios, iTeos, IgM, Trillium, Invaria, GV20, Bioentre, and Geode. A.H. Sharpe reports grants from NIH P01 AI056299, NIH P50 CA101942, NIH P01 CA236749, NIH U54 CA224088, and R01CA276866 during the conduct of the study; grants from Vertex, Moderna, Merck Sharp and Dohme, AbbVie, Quark/IOME, Roche, Erasca, TaiwanBio, and Calico, personal fees from Surface Oncology, Sqz Biotechnologies, Elpiscience, Selecta, Bicara, Fibrogen, Alixia, other support from Monopteros, GlaxoSmith Kline, Janssen, Amgen, Corner Therapeutics, and personal fees from Bioentre outside the submitted work; in addition, A.H. Sharpe has a patent 7,432,059 with royalties paid from Roche, Merck, Bristol-Myers-Squibb, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, Leica, Mayo Clinic, Dako, and Novartis, a patent 7722868 with royalties paid from Roche, Merck, Bristol-Myers-Squibb, EMD-Serono, Boehringer-Ingelheim, AstraZeneca, Leica, Mayo Clinic, Dako, and Novartis, a patent 8652465 licensed to Roche, a patent 9457080 licensed to Roche, a patent 9683048 licensed to Novartis, a patent 9815898 licensed to Novartis, a patent 9845356 licensed to Novartis, a patent 10202454 licensed to Novartis, a patent 10457733 licensed to Novartis, a patent 9580684 issued, a patent 9988452 issued, a patent 10370446 issued, a patent 10457733 issued, a patent 10752687 issued, a patent 10851165 issued, and a patent 10934353 issued; and A.H. Sharpe is on scientific advisory boards for the Massachusetts General Cancer Center, Program in Cellular and Molecular Medicine at Boston Children’s Hospital, the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, Perlmutter Cancer Center at NYU, the Gladstone Institutes and the Johns Hopkins Bloomberg-Kimmel Institute for Cancer Immunotherapy. She is an academic editor for the
Figures



References
-
- Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg 2016;103:e29–46. - PubMed
-
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell 2023;186:243–78. - PubMed
-
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018;14:576–90. - PubMed
MeSH terms
Grants and funding
- K22CA266150/National Cancer Institute (NCI)
- Banting Postdoctoral Fellowship from the Canadian Institutes of Health Research
- P01 CA236749/CA/NCI NIH HHS/United States
- P01 AI056299/AI/NIAID NIH HHS/United States
- F31 CA281090/CA/NCI NIH HHS/United States
- P50 CA101942/CA/NCI NIH HHS/United States
- R01 CA276866/CA/NCI NIH HHS/United States
- Ludwig Center at Harvard (Ludwig Center)
- T32 CA009172/CA/NCI NIH HHS/United States
- F32 CA247072/CA/NCI NIH HHS/United States
- 130373-PF-17-132-01-CCG/American Cancer Society (ACS)
- 5F31CA224601/National Institutes of Health (NIH)
- U01 CA267827/CA/NCI NIH HHS/United States
- U54 CA225088/CA/NCI NIH HHS/United States
- K08 CA248960/CA/NCI NIH HHS/United States
- F31 CA224601/CA/NCI NIH HHS/United States
- K22 CA266150/CA/NCI NIH HHS/United States
- Paul F. Glenn Foundation for Medical Research
- U54 CA224088/National Institutes of Health (NIH)
- F32-CA247072/National Cancer Institute (NCI)
- Cell Biology Education and Fellowship Fund at Harvard Medical School
- K08CA248960/National Institutes of Health (NIH)
- Burroughs Wellcome Fund Career Award for Medical Scientists
- F31CA281090/National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Research Materials

