Study protocol for Sauti ya Vijana (The Voice of Youth): A hybrid-type 1 randomized trial to evaluate effectiveness and implementation of a mental health and life skills intervention to improve health outcomes for Tanzanian youth living with HIV
- PMID: 39186768
- PMCID: PMC11346953
- DOI: 10.1371/journal.pone.0305471
Study protocol for Sauti ya Vijana (The Voice of Youth): A hybrid-type 1 randomized trial to evaluate effectiveness and implementation of a mental health and life skills intervention to improve health outcomes for Tanzanian youth living with HIV
Abstract
Objective: Young people living with HIV (YPLWH) experience increased morbidity and mortality compared to all other age groups. Adolescence brings unique challenges related to sexual reproductive health, the elevated importance of peer groups, and often, emerging symptoms of emotional distress. Failure to address this unique life stage for YPLWH can lead to worse HIV and mental health outcomes. Herein lies the protocol for a hybrid-type-1 effectiveness-implementation trial designed to evaluate a mental health and life skills intervention that aims to address these needs for YPLWH in Tanzania.
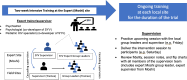
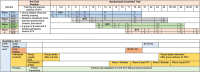
Methods: This is an individually randomized group-treatment trial designed to evaluate the effectiveness of Sauti ya Vijana (SYV: The Voice of Youth) toward improving viral suppression (HIV RNA <400 copies/mL) and mental health outcomes and to assess implementation including acceptability, feasibility, fidelity, and cost-effectiveness of the manualized intervention. The trial is being conducted across four geographically distinct regions of Tanzania. Peer group leaders (PGL) with lived HIV experience deliver the 10-session group-based intervention and two individual sessions during which participants describe their disclosure narrative (when they learned they live with HIV) and value-based goal setting. Caregiver or chosen supportive adults are encouraged to attend two specific group sessions with their youth. Participants are 10-24 years of age, prescribed antiretroviral therapy for at least 6 months, fully aware of their HIV status, able to commit to session attendance, and able to understand and meaningfully contribute to group sessions. Participant study visits occur at 5 time points for evaluation: baseline, 4-, 6-, 12-, and 18-months post baseline. A single booster session is conducted before the 12-month visit. Study visits evaluate mental health, adverse childhood events, interpersonal violence, resilience, stigma, HIV knowledge, substance use, sexual relationships, ART adherence, and HIV RNA. Implementation outcomes evaluate feasibility and acceptability through attendance, intervention session notes, focus discussion groups and qualitative interviews. Fidelity to the intervention is measured using fidelity checklists by a PGL observer at each group session. Cost effectiveness is calculated using an incremental cost-effectiveness ratio that utilizes a patient cost questionnaire and financial records of study costs.
Significance: Few mental health interventions for YPLWH have demonstrated effectiveness. Results from this study will provide information about effectiveness and implementation of a peer-led intervention for delivering a mental health and life skills intervention in low-income settings.
Trial identifier: This trial is registered at clinicaltrials.gov NCT05374109.
Copyright: © 2024 Mollel et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
All authors have declared that no competing interests exist.
Figures




References
-
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). The Youth Bulge and HIV. Available at www.unaids.org/en/resources/documents/2018/the-youth-bulge-and-hiv. Last accessed 28 November 2019.
-
- UNAIDS. Youth and HIV Mainstreaming a three-lens approach to youth participation. Accessed on 17 August 2018. Accessed at http://www.unaids.org/en/resources/documents/2018/youth-and-hiv. 2018.
-
- White AM. Understanding adolescent brain development and its implications for the clinician. Adolesc Med State Art Rev. 2009;20(1):73–90, viii-ix. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

