Suppressed HIV antibody responses following exposure to antiretrovirals-evidence from PrEP randomized trials and early antiretroviral treatment initiation studies
- PMID: 39186969
- PMCID: PMC11569788
- DOI: 10.1016/j.ijid.2024.107222
Suppressed HIV antibody responses following exposure to antiretrovirals-evidence from PrEP randomized trials and early antiretroviral treatment initiation studies
Abstract
Background: Exposure to antiretrovirals at or early after HIV acquisition can suppress viral replication and blunt antibody (Ab) responses; a reduced HIV detectability could impact diagnosis and blood donation screening.
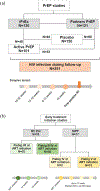
Methods: We used three antigen (Ag)/Ab assays and one nucleic acid test (NAT) to analyze samples collected in pre-exposure prophylaxis (PrEP) trials (iPrEx; Partners PrEP) before infection detection by Ab-only rapid diagnostic tests (RDTs), and in early antiretroviral treatment (ART) initiation studies (RV254; SIPP).
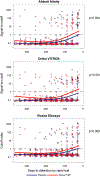
Results: Reactivity using NAT and Ag/Ab assays in samples collected up to 8 weeks prior to the first reactive RDT from 251 PrEP trials participants varied between 49-61% for active PrEP users and between 27-37% for placebo users. Among RV254 participants, reactivity in Ag/Ab assays was <100% at all timepoints, and lower among those initiating ART earlier. Seroreversions occurred for 29% (16/55), and blood donation screening with NAT and Ag/Ab assays could have missed up to 36% (20/55) of RV254 participants. For SIPP participants, who started ART at later timepoints, Ag/Ab assays identified infections with no evidence of reactivity waning.
Conclusion: PrEP and early ART initiation can delay or reduce HIV detectability. Considerations for the implementation of NAT and Ag/Ab tests in PrEP/PEP programs relying on Ab-only RDTs should be balanced according to feasibility and public health impact. While blood transfusion services using Ab-only RDTs for HIV screening should adopt higher sensitivity tests, surveillance and further research are needed to determine the need for novel HIV testing algorithms for those already using NAT and Ag/Ab screening assays.
Keywords: Antiretroviral therapy; Delayed diagnosis; Diagnostics; HIV testing; Pre-exposure prophylaxis; Serologic tests.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: SF has received consulting support from Gilead Sciences for unrelated work. MPB has received research support from Abbott, Grifols, Roche, and QuidelOrtho. He received no personal compensation, equity, advisory committee role or travel support. MLM is an employee and shareholder of Gilead Sciences. BC received research funding and reagents from Hologic and from Grifols Diagnostic Solutions and has been part of the speakers´ bureau of Abbott Inc. SB is an employee and received honoraria for lectures from Grifols Diagnostic Solutions.
Figures


References
-
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003;17(13):1871–9. - PubMed
-
- Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time until emergence of HIV test reactivity following infection with HIV-1: implications for interpreting test results and retesting after exposure. Clin Infect Dis 2017;64(1):53–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

