Hyperglycemic environments directly compromise intestinal epithelial barrier function in an organoid model and hyaluronan (∼35 kDa) protects via a layilin dependent mechanism
- PMID: 39187208
- PMCID: PMC12327694
- DOI: 10.1016/j.matbio.2024.08.007
Hyperglycemic environments directly compromise intestinal epithelial barrier function in an organoid model and hyaluronan (∼35 kDa) protects via a layilin dependent mechanism
Abstract
Background: Metabolic syndrome and diabetes in obese individuals are strong risk factors for development of inflammatory bowel disease (IBD) and colorectal cancer. The pathogenic mechanisms of low-grade metabolic inflammation, including chronic hyperglycemic stress, in disrupting gut homeostasis are poorly understood. In this study, we sought to understand the impact of a hyperglycemic environment on intestinal barrier integrity and the protective effects of small molecular weight (35 kDa) hyaluronan on epithelial barrier function.
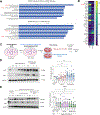
Methods: Intestinal organoids derived from mouse colon were grown in normal glucose media (5 mM) or high glucose media (25 mM) to study the impact of hyperglycemic stress on the intestinal barrier. Additionally, organoids were pretreated with 35 kDa hyaluronan (HA35) to investigate the effect of hyaluronan on epithelial barrier under high glucose stress. Immunoblotting as well as confocal imaging was used to understand changes in barrier proteins, quantitative as well as spatial distribution, respectively. Alterations in barrier function were measured using trans-epithelial electrical resistance and fluorescein isothiocyanate flux assays. Untargeted proteomics analysis was performed to elucidate mechanisms by which HA35 exerts a protective effect on the barrier. Intestinal organoids derived from receptor knockout mice specific to various HA receptors were utilized to understand the role of HA receptors in barrier protection under high glucose conditions.
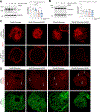
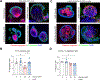
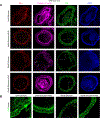
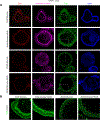
Results: We found that high glucose stress decreased the protein expression as well as spatial distribution of two key barrier proteins, zona occludens-1 (ZO-1) and occludin. HA35 prevented the degradation or loss of ZO-1 and maintained the spatial distribution of both ZO-1 and occludin under hyperglycemic stress. Functionally, we also observed a protective effect of HA35 on the epithelial barrier under high glucose conditions. We found that HA receptor, layilin, was involved in preventing barrier protein loss (ZO-1) as well as maintaining spatial distribution of ZO-1 and occludin. Additionally, proteomics analysis showed that cell death and survival was the primary pathway upregulated in organoids treated with HA35 under high glucose stress. We found that XIAP associated factor 1 (Xaf1) was modulated by HA35 thereby regulating apoptotic cell death in the intestinal organoid system. Finally, we observed that spatial organization of both focal adhesion kinase (FAK) as well as F-actin was mediated by HA35 via layilin.
Conclusion: Our results highlight the impact of hyperglycemic stress on the intestinal barrier function. This is of clinical relevance, as impaired barrier function has been observed in individuals with metabolic syndrome. Additionally, we demonstrate barrier protective effects of HA35 through its receptor layilin and modulation of cellular apoptosis under high glucose stress.
Keywords: Apoptosis; Hyaluronan; Hyperglycemia; Intestinal barrier; Layilin; Metabolic syndrome; Organoids.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

