Development of specialized devices for microbial experimental evolution
- PMID: 39187274
- PMCID: PMC11482599
- DOI: 10.1111/dgd.12940
Development of specialized devices for microbial experimental evolution
Abstract
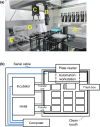
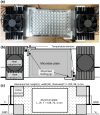
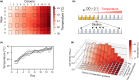
Experimental evolution of microbial cells provides valuable information on evolutionary dynamics, such as mutations that contribute to fitness gain under given selection pressures. Although experimental evolution is a promising tool in evolutionary biology and bioengineering, long-term culture experiments under multiple environmental conditions often impose an excessive workload on researchers. Therefore, the development of automated systems significantly contributes to the advancement of experimental evolutionary research. This review presents several specialized devices designed for experimental evolution studies, such as an automated system for high-throughput culture experiments, a culture device that generate a temperature gradient, and an automated ultraviolet (UV) irradiation culture device. The ongoing development of such specialized devices is poised to continually expand new frontiers in experimental evolution research.
Keywords: automation; experimental evolution; microorganisms; temperature control; ultraviolet irradiation.
© 2024 The Author(s). Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

