Aberrant mitochondrial DNA synthesis in macrophages exacerbates inflammation and atherosclerosis
- PMID: 39187565
- PMCID: PMC11347661
- DOI: 10.1038/s41467-024-51780-1
Aberrant mitochondrial DNA synthesis in macrophages exacerbates inflammation and atherosclerosis
Abstract
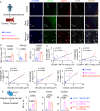
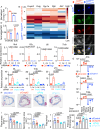
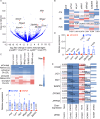
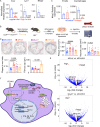
There is a large body of evidence that cellular metabolism governs inflammation, and that inflammation contributes to the progression of atherosclerosis. However, whether mitochondrial DNA synthesis affects macrophage function and atherosclerosis pathology is not fully understood. Here we show, by transcriptomic analyzes of plaque macrophages, spatial single cell transcriptomics of atherosclerotic plaques, and functional experiments, that mitochondrial DNA (mtDNA) synthesis in atherosclerotic plaque macrophages are triggered by vascular cell adhesion molecule 1 (VCAM-1) under inflammatory conditions in both humans and mice. Mechanistically, VCAM-1 activates C/EBPα, which binds to the promoters of key mitochondrial biogenesis genes - Cmpk2 and Pgc1a. Increased CMPK2 and PGC-1α expression triggers mtDNA synthesis, which activates STING-mediated inflammation. Consistently, atherosclerosis and inflammation are less severe in Apoe-/- mice lacking Vcam1 in macrophages. Downregulation of macrophage-specific VCAM-1 in vivo leads to decreased expression of LYZ1 and FCOR, involved in STING signalling. Finally, VCAM-1 expression in human carotid plaque macrophages correlates with necrotic core area, mitochondrial volume, and oxidative damage to DNA. Collectively, our study highlights the importance of macrophage VCAM-1 in inflammation and atherogenesis pathology and proposes a self-acerbating pathway involving increased mtDNA synthesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

