Stearoyl-CoA desaturase inhibition is toxic to acute myeloid leukemia displaying high levels of the de novo fatty acid biosynthesis and desaturation
- PMID: 39187579
- PMCID: PMC11518998
- DOI: 10.1038/s41375-024-02390-9
Stearoyl-CoA desaturase inhibition is toxic to acute myeloid leukemia displaying high levels of the de novo fatty acid biosynthesis and desaturation
Abstract
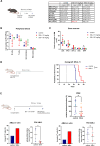
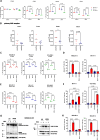
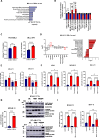
Identification of specific and therapeutically actionable vulnerabilities, ideally present across multiple mutational backgrounds, is needed to improve acute myeloid leukemia (AML) patients' outcomes. We identify stearoyl-CoA desaturase (SCD), the key enzyme in fatty acid (FA) desaturation, as prognostic of patients' outcomes and, using the clinical-grade inhibitor SSI-4, show that SCD inhibition (SCDi) is a therapeutic vulnerability across multiple AML models in vitro and in vivo. Multiomic analysis demonstrates that SCDi causes lipotoxicity, which induces AML cell death via pleiotropic effects. Sensitivity to SCDi correlates with AML dependency on FA desaturation regardless of mutational profile and is modulated by FA biosynthesis activity. Finally, we show that lipotoxicity increases chemotherapy-induced DNA damage and standard chemotherapy further sensitizes AML cells to SCDi. Our work supports developing FA desaturase inhibitors in AML while stressing the importance of identifying predictive biomarkers of response and biologically validated combination therapies to realize their full therapeutic potential.
© 2024. The Author(s).
Conflict of interest statement
LH is CEO and co-founder of Modulation Therapeutics, the company holding intellectual property to SSI-4. J.A.C. holds a patent regarding the use of the SCD inhibitor SSI-4.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

